| 生物活性 | |||
|---|---|---|---|
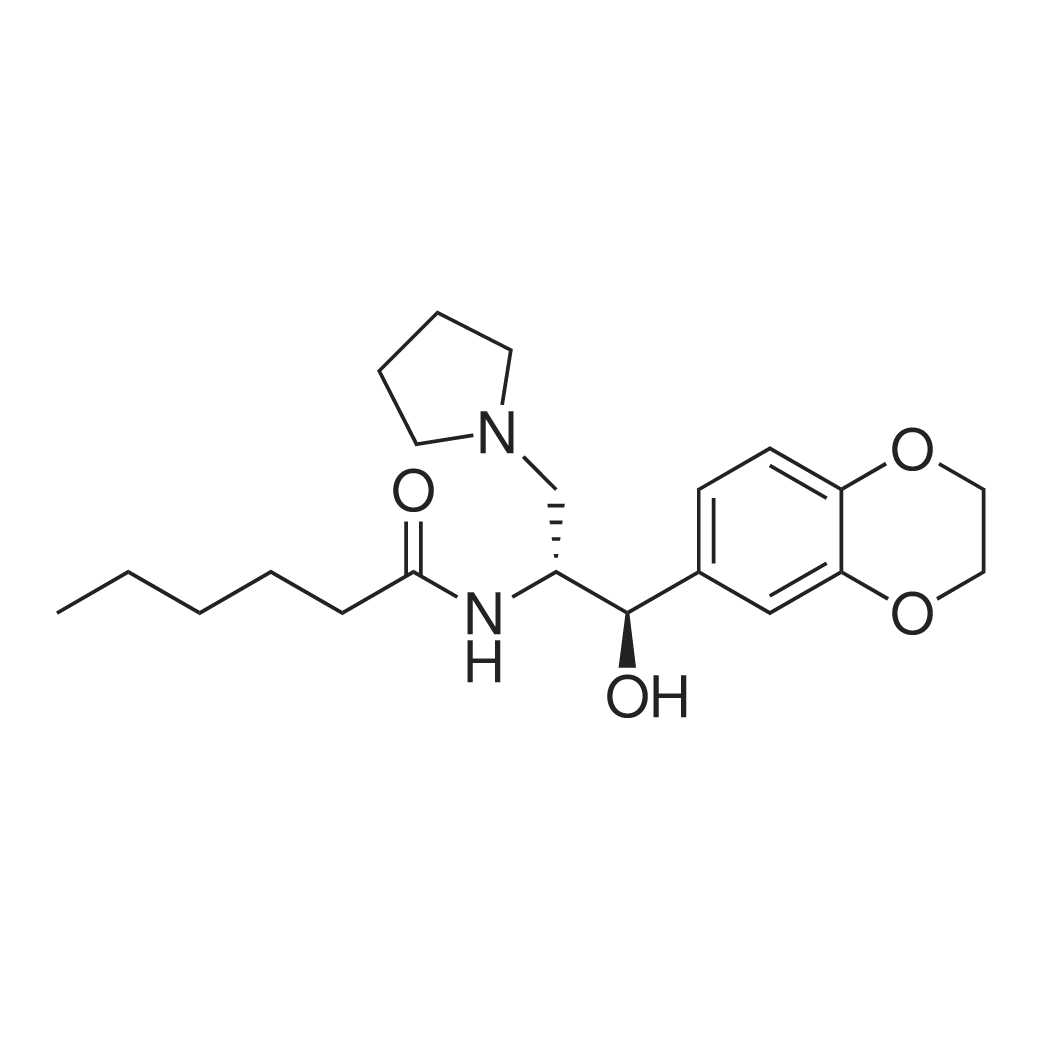
| 描述 | Eliglustat is a selective inhibitor of glucosylceramide synthase that is extensively metabolized by CYP2D6 and, to a lesser extent by CYP3A4; it is also an inhibitor of the P-gp transporter[3]. Eliglustat is an oral inhibitor of glucosylceramide synthase which is used in the therapy of type 1 Gaucher disease[4]. Eliglustat inhibits glucosylceramide synthase, thereby decreasing production of the substrate glucosylceramide and reducing its accumulation[5]. Administration of eliglustat to Swiss CD-1 mice at 0, 10, 25 or 75 mg/kg/day for 104 weeks by dietary admixture did not influence survival or bodyweight evolution, or produce any clinical indication of poor condition. Eliglustat was not carcinogenic to mice or rats in standard lifetime bioassays[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01074944 | Gaucher Disease | Phase 3 | Completed | - | - |
| NCT00358150 | Gaucher Disease, Type 1 ... 展开 >> Cerebroside Lipidosis Syndrome Glucocerebrosidase Deficiency Disease Glucosylceramide Beta-Glucosidase Deficiency Disease Gaucher Disease, Non-Neuronopathic Form 收起 << | Phase 2 | Completed | - | United States, New York ... 展开 >> New York University New York, New York, United States New York, New York, United States Argentina Aprillus Asistencia e Investigación Buenos Aires, Argentina Hospital de Oncologia Maria Curie Buenos Aires, Argentina IMAI Buenos Aires, Argentina Instituto Argentino de Diagnostico y Tratamiento (IADT) Buenos Aires, Argentina Buenos Aires, Argentina Hospital Ramos Mejia Ciudad Autonoma de Buenos Aires, Argentina Israel Rambam Medical Center Haifa, Israel Haifa, Israel Sha'are Zedek Medical Centre Jerusalem, Israel Jerusalem, Israel Italy Universita degli Studi di Milano Milano, Italy Mexico Instituto Mexicano del Seguro Social D.f., Mexico Mexico City, Mexico Russian Federation Hematology Research Center of Ministry of Healthcare of the Russian Federation Moscow, Russian Federation Moscow, Russian Federation 收起 << |
| NCT00943111 | - | Completed | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.47mL 0.49mL 0.25mL |
12.36mL 2.47mL 1.24mL |
24.72mL 4.94mL 2.47mL |
| 参考文献 |
|---|