产品说明书
| 生物活性 | |||
|---|---|---|---|
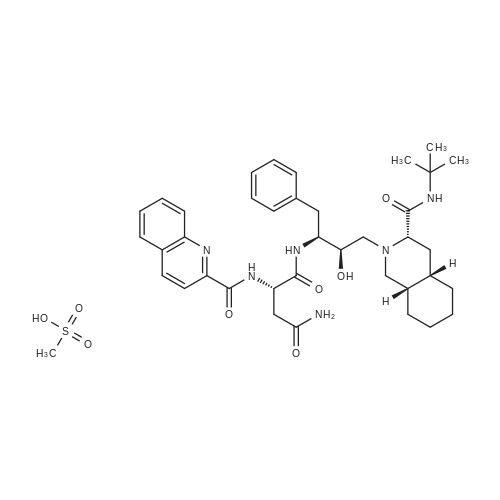
| 描述 | Saquinavir (SAQ) mesylate is a potent inhibitor of the HIV-1 protease indicated in combination with other antiretrovirals for the management of HIV-1 infection[3]. Saquinavir is an anti-retroviral drug with very low oral bioavailability (e.g. 0.7-4.0%) due to its affinity toward efflux transporters (P-gp) and metabolic enzymes (CYP3A4)[4]. As with other HIV protease inhibitors, saquinavir inhibits the cleavage of the gag-pol protein substrate leading to the release of structurally defective and functionally inactive viral particles. It is active on both HIV-1 and HIV-2, and also has activity on chronically infected cells and HIV strains resistant to reverse transcriptase inhibitors. Saquinavir is characterised by a low bioavailability which is further reduced in the fasting state. Because of its metabolic interference with the CYP system, saquinavir cannot be coadministered with astemizole, terfenadine or cisapride. Saquinavir is generally well tolerated, with mild gastrointestinal symptoms representing the most commonly observed adverse effects[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00035932 | HIV Infections | Phase 3 | Completed | - | - |
| NCT00197145 | Infection, Human Immunodeficie... 展开 >>ncy Virus I 收起 << | Phase 3 | Terminated | - | - |
| NCT00197145 | - | Terminated | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.30mL 0.26mL 0.13mL |
6.52mL 1.30mL 0.65mL |
13.04mL 2.61mL 1.30mL |
| 参考文献 |
|---|