| 生物活性 | |||
|---|---|---|---|
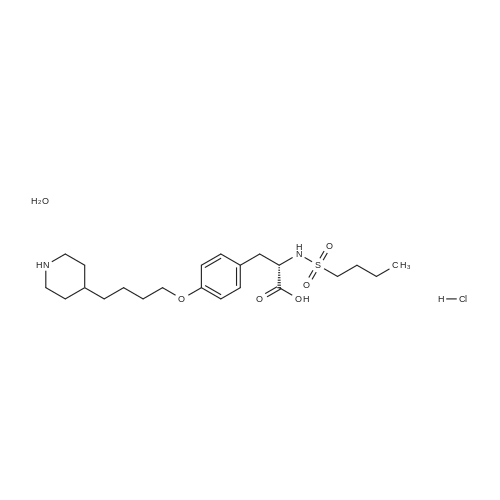
| 描述 | The binding of glycoprotein (GP) IIb/IIIa receptor to fibrinogen is a prerequisite in platelet activation and aggregation. Tirofiban Hydrochloride Monohydrate is the hydrochloride monohydrate form of Tirofiban. Tirofiban is a potent GPIIb/IIIa antagonist. It inhibited the aggregation of in vitro human gel-filtered platelets induced by ADP, collagen, gamma-thrombin, and U46619 with IC50 values ranging from 12 nM to 31 nM. It also displayed inhibitory activity against human PRP platelet aggregation induced by ADP, collagen, gamma-thrombin, U46619, epinephrine, and arachidonic acid with IC50 values ranging from 31 nM to 66 nM. Tirofiban inhibited the platelet aggregation in human, rhesus monkey, and dog whole blood with IC50 values of 81, 140, and 353 nM, respectively. The intravenous administration of tirofiban (1 mg/kg) in dogs significantly inhibited ex vivo ADP-induced platelet aggregation in platelet-rich plasma (PRP) and the prolongation of template bleeding time. Continuous infusions of tirofiban (0.1-10 µg/kg/min) inhibited ex vivo platelet aggregation in PRP induced by ADP and collagen in a dose-dependent manner. Moreover, the combined treatment with tirofiban (1µg/kg/min, i.v., 120min) and ticlopidine (20 mg/kg/day, p.o., 4 days) in dogs did not change the pharmacokinetic profile of tirofiban nor the platelet count[3]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01087723 | Acute Coronary Syndrome | Phase 3 | Completed | - | - |
| NCT01087723 | - | Completed | - | - | |
| NCT01103440 | Stable Angina | Phase 2 | Completed | - | United States, New York ... 展开 >> Mount Sinai Medical Center New York, New York, United States, 10029 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.02mL 0.40mL 0.20mL |
10.10mL 2.02mL 1.01mL |
20.20mL 4.04mL 2.02mL |
| 参考文献 |
|---|