| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
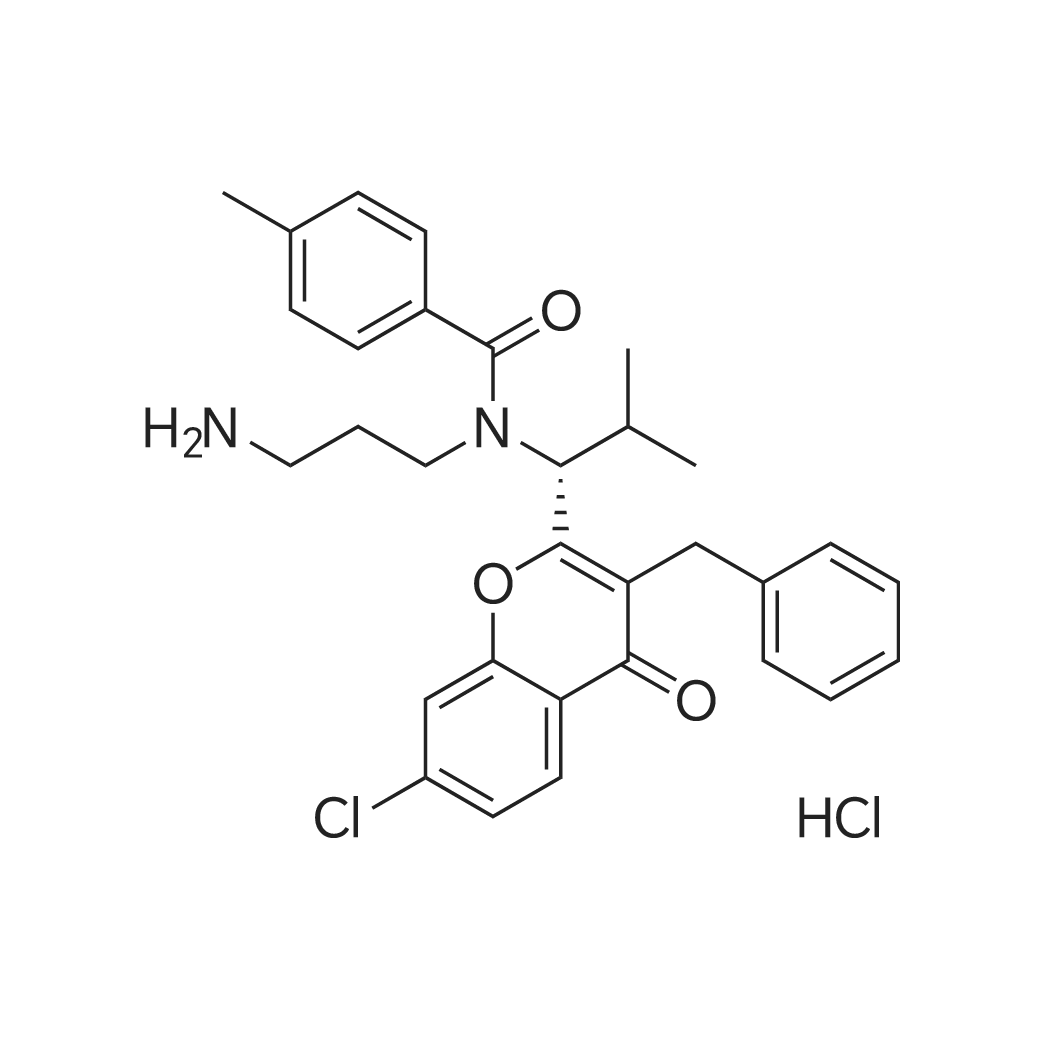
| 描述 | SB-743921 is a second generation, highly potent and active KSP inhibitor with Ki of 0.1 nM, showing no affinity to MKLP1, Kin2, Kif1A, Kif15, KHC, Kif4 and CENP-E. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00136513 | Solid Tumor Cancer | Phase 1 | Completed | - | United States, Pennsylvania ... 展开 >> GSK Investigational Site Pittsburgh, Pennsylvania, United States, 15232 United States, Wisconsin GSK Investigational Site Madison, Wisconsin, United States, 53792 收起 << |
| NCT00343564 | Non-Hodgkin's Lymphoma ... 展开 >> Hodgkin's Disease 收起 << | Phase 1 Phase 2 | Completed | - | United States, New Jersey ... 展开 >> Hackensack University Medical Center Hackensack, New Jersey, United States, 07601 United States, New York Cornell University Medical Center New York, New York, United States, 10021 Memorial Sloan-Kettering Caner Center New York, New York, United States, 10021 Herbert Irving Comprehensive Cancer Center New York, New York, United States, 10032 United States, North Carolina University of North Carolina Chapel Hill, North Carolina, United States, 27599 United States, Tennessee Sarah Cannon Cancer Research Institute Nashville, Tennessee, United States, 37203 Russian Federation Russian Medical Academy of Postgraduate Education Moscow, Russian Federation, 115478 St. Petersburg State PAVLOV Medical University Saint Petersburg, Russian Federation, 197002 收起 << |
| NCT00343564 | - | Completed | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.81mL 0.36mL 0.18mL |
9.03mL 1.81mL 0.90mL |
18.07mL 3.61mL 1.81mL |
| 参考文献 |
|---|