| 生物活性 | |||
|---|---|---|---|
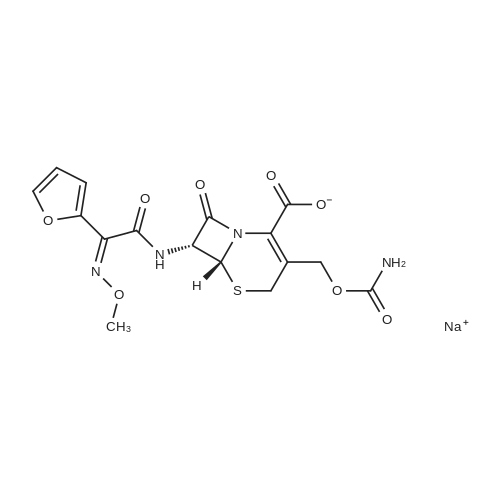
| 描述 | Cefuroxime sodium is an orally active second-generation cephalosporin antibiotic with increased stability to β-lactamase. It is against Staphylococcus aureus methicillin susceptible; S. aureus, methicillin resistant; Streptococcus pyogenes; S. pneumonia; S. viridans; S. faecalis and Clostridium spp with MIC values of 0.25 μg/ml, 5.9 μg/ml, 0.125 μg/ml, 0.125 μg/ml, 0.125 μg/ml, >125.0 μg/ml, and 1.2 μg/ml, respectively. It is highly active against Neisseria gonorrhoeae, Neisseria meningitidis, and also Haemophilus influenzae, including ampicillin-resistant strains. Cefuroxime is rapidly bactericidal and induces the formation and subsequent lysis of filamentous forms over a small concentration range[2]. Rabbits (weighing 2.0 to 2.5 kg) are challenged intravenously with S. aureus strain 630 (a penicillinase-producing strain), the median effective dose of Cefuroxime sodium is 3 mg/kg as a result of the protection test[3]. After both i.v. and intramuscular injections of cefuroxime sodium, the concentrations of cefuroxime in urine were much higher than that in serum. The systemic bioavailability of cefuroxime sodium in goats after intramuscular injections of 20 and 40 mg/kg body weight was 88.4 percent and 103.5 percent, respectively. In vitro protein binding of cefuroxime sodium in goat's serum was low, ranging from 13.3 percent to 21.6 percent with an average of 17.0 percent[4]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03216642 | - | Recruiting | April 17, 2018 | China, Guangdong ... 展开 >> Clinical Research Center Recruiting Guangzhou, Guangdong, China, 510000 Contact: Wang Zhichong, M.D. 02087333391 wzc001@hotmail.com Contact: Liu Chengxiu 02087333391 13760787745@163.com 收起 << | |
| NCT00355602 | Colitis, Ulcerative | Not Applicable | Completed | - | United Kingdom ... 展开 >> Ninewells Hospital and Medical School Dundee, Angus, United Kingdom, DD1 9SY 收起 << |
| NCT02171338 | Pneumonia Acu... 展开 >>te Exacerbation of Chronic Obstructive Airways Disease 收起 << | Phase 4 | Unknown | September 2014 | Denmark ... 展开 >> Holbæk Hospital Holbæk, Denmark, 4300 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.24mL 0.45mL 0.22mL |
11.20mL 2.24mL 1.12mL |
22.40mL 4.48mL 2.24mL |
| 参考文献 |
|---|