| 生物活性 | |||
|---|---|---|---|
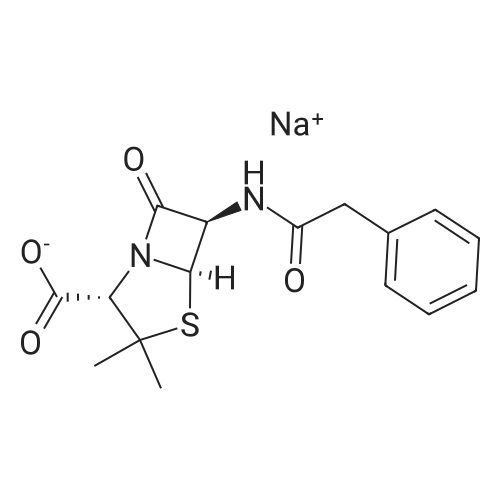
| 描述 | Penicillin G sodium is a typical β-lactam antibiotic. Intravenous administration of a total dose of more than 200 million IU of penicillin-G led to sensitisation of lymphocytes and formation of specific anti-penicilloyl antibodies of the IgG class[2]. Intrapartum penicillin G prophylaxis aims to prevent early-onset group B streptococci (GBS) sepsis by interrupting vertical transmission[3]. High-dose intravenous therapy with penicillin-G always results in both sensitised lymphocytes and rise of anti-penicilloyl IgG antibodies. Strict application of freshly prepared single doses prevents the majority of adverse reactions following highdose intravenous penicillin-G therapy[4]. The addition of citrate buffer to a penicillin G preparation for injection was in a preliminary study found to improve the local tolerance in rabbits. The local tolerance may influence the serum levels in a way that may influence the penetration of penicillin[5]. Most Gram-negative urinary pathogens are sensitive to 5-50 mug of penicillin G per ml. There is a rational basis for the use of oral penicillin G to treat urinary infection due to Gram-negative bacilli[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.81mL 0.56mL 0.28mL |
14.03mL 2.81mL 1.40mL |
28.06mL 5.61mL 2.81mL |
| 参考文献 |
|---|
|
[6]Hulbert J. Urinary tract infection and oral penicillin G. J Clin Pathol. 1972 Jan;25(1):73-5 |