| 生物活性 | |||
|---|---|---|---|
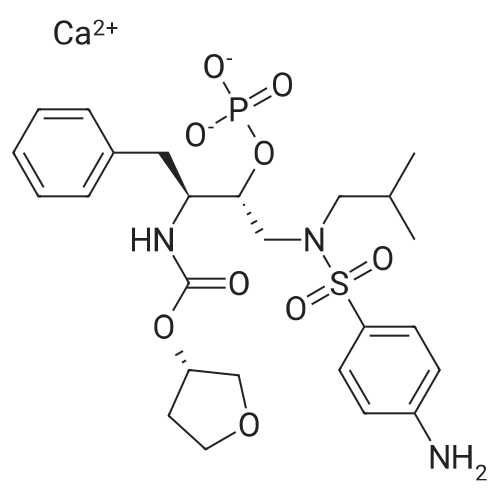
| 描述 | Newly released human immunodeficiency virus type 1 (HIV-1) particles obligatorily undergo a maturation process to become infectious. The HIV-1 protease initiates this step, catalyzing the cleavage of the Gag and Gag-Pro-Pol structural polyproteins[3]. Fosamprenavir, the phosphate ester prodrug of the HIV-1 PI (protease inhibitor) amprenavir, is one of the most recently approved HIV-1 PIs. The availability of fosamprenavir allows for substantial reductions in pill number and pill size, and omits the need for food and fluid requirements that are associated with some of the other HIV-1 Pis. Three fosamprenavir dosage regimens are approved for the treatment of HIV-1 PI-naive patients, including fosamprenavir 1,400 mg twice daily, fosamprenavir 1,400 mg once daily plus ritonavir 200 mg once daily, and fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily. Plasma amprenavir concentrations are quantifiable within 15 minutes of dosing and peak at 1.5-2 hours after fosamprenavir dosing[4]. Fosamprenavir has been compared with amprenavir in a 6-week randomised controlled trial in HIV-infected adults. Amprenavir 1200 mg twice daily was compared with fosamprenavir 1395 mg twice daily or 1860 mg twice daily. Peak plasma amprenavir concentrations were 30% lower with fosamprenavir, AUCs were equivalent, and trough concentrations were increased by 28% with fosamprenavir 1395 mg twice daily and 46% with fosamprenavir 1860 mg twice daily[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03290131 | Multiple Sclerosis ... 展开 >> Spasticity, Muscle 收起 << | Phase 3 | Recruiting | April 2019 | United States, Arizona ... 展开 >> Xenoscience Inc. Recruiting Phoenix, Arizona, United States, 85004 United States, California Neuro-Pain Medical Center Recruiting Fresno, California, United States, 93710 United States, Florida Meridien Research Recruiting Tampa, Florida, United States, 33634 收起 << |
| NCT03222349 | Epilepsy | Phase 2 | Recruiting | November 24, 2018 | United States, Arizona ... 展开 >> University of Arizona Recruiting Tucson, Arizona, United States, 85719 Contact: Sejal Jain United States, Florida NW FL Clinical Research Group, LLC Recruiting Gulf Breeze, Florida, United States, 32561 Contact: Weldon Mauney United States, New Jersey Children's St. Peters University Hospital Recruiting New Brunswick, New Jersey, United States, 08901 Contact: Carlos Lastra United States, New York Icahn School of Medicine at Mount Sinai Recruiting New York, New York, United States, 10029 Contact: Harriet Kang, MD 914-428-0529 Principal Investigator: Harriet Kang, MD Sub-Investigator: Patricia McGoldrick, NP Sub-Investigator: Steven Wolf, MD University of Rochester Recruiting Rochester, New York, United States, 14607 Contact: Inna Hughes United States, North Carolina Onsite Clinical Solutions LLC Recruiting Charlotte, North Carolina, United States, 28203 Contact: Robert Nahouraii United States, Pennsylvania Children's Hospital of Philadelphia Recruiting Philadelphia, Pennsylvania, United States, 19104 Contact: Dennis Dlugos United States, Texas Dell Children's Medical Center Recruiting Austin, Texas, United States, 78723 Contact: David Clarke Austin Epilepsy Care Center Recruiting Austin, Texas, United States, 78758 Contact: Sami Aboumatar, MD 512-339-8831 sami@northaustinneuro.com United States, Virginia Virginia Commonwealth University Recruiting Richmond, Virginia, United States, 23298 Contact: Syndi Seinfeld 收起 << |
| NCT03179891 | Epilepsy | Phase 2 | Recruiting | April 29, 2019 | United States, Arizona ... 展开 >> Arizona Health Sciences Center Recruiting Tucson, Arizona, United States, 85724-5023 Contact: David M. Labiner, MD 520-626-2006 United States, California Rancho Research Institute Recruiting Downey, California, United States, 90242 Contact: Hui Gong, MD 818-822-4916 hgong2@dhs.lacounty.gov United States, Connecticut Yale University School of Medicine-Comprehensive Epilepsy Center Recruiting New Haven, Connecticut, United States, 06520-8018 Contact: Kamil Detyniecki, MD 203-785-3865 kamil.detyniecki@yale.edu United States, Hawaii Hawaii Pacific Neuroscience Recruiting Honolulu, Hawaii, United States, 96817 Contact: Kore Liow, MD 808-261-4476 kliow@hawaiineuroscience.com United States, Maryland Mid-Atlantic Epilepsy and Sleep Center Recruiting Bethesda, Maryland, United States, 20817 Contact: Pavel Klein, M.B. 301-530-9744 kleinp@epilepsydc.com Contact: Diep Bui, M.D. United States, New Jersey Saint Peter's University Hospital Recruiting New Brunswick, New Jersey, United States, 08901 Contact: Carlos Lastra, MD 732-745-8600 clastra@saintpetersuh.com United States, New York University of Rochester Medical Center Recruiting Rochester, New York, United States, 14642 Contact: Trenton J. Tollefson, MD 507-273-4872 trenton_tollefson@urmc.rochester.edu United States, North Carolina Onsite Clinical Solutions LLC Recruiting Charlotte, North Carolina, United States, 28203 Contact: Robert Ataollah Nahouraii United States, Pennsylvania Hospital of the University of Pennsylvania Recruiting Philadelphia, Pennsylvania, United States, 19104 Contact: Michael A. Gelfand, MD, PhD 215-349-5166 michael.gelfand@uphs.upenn.edu United States, Texas Austin Epilepsy Care Center Recruiting Austin, Texas, United States, 78758 Contact: Sami Aboumatar, MD 512-339-8831 sami@northaustinneuro.com United States, Virginia Virginia Commonwealth University Medical Center Recruiting Richmond, Virginia, United States, 23219 Contact: Syndi A. Seinsefl, DO 804-828-0445 syndi.seinfeld@vcuhealth.org 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.60mL 0.32mL 0.16mL |
8.02mL 1.60mL 0.80mL |
16.03mL 3.21mL 1.60mL |
| 参考文献 |
|---|
|
[3]The Triple Threat of HIV-1 Protease Inhibitors [4]Fosamprenavir : Clinical Pharmacokinetics and Drug Interactions of the Amprenavir Prodrug |