| 生物活性 | |||
|---|---|---|---|
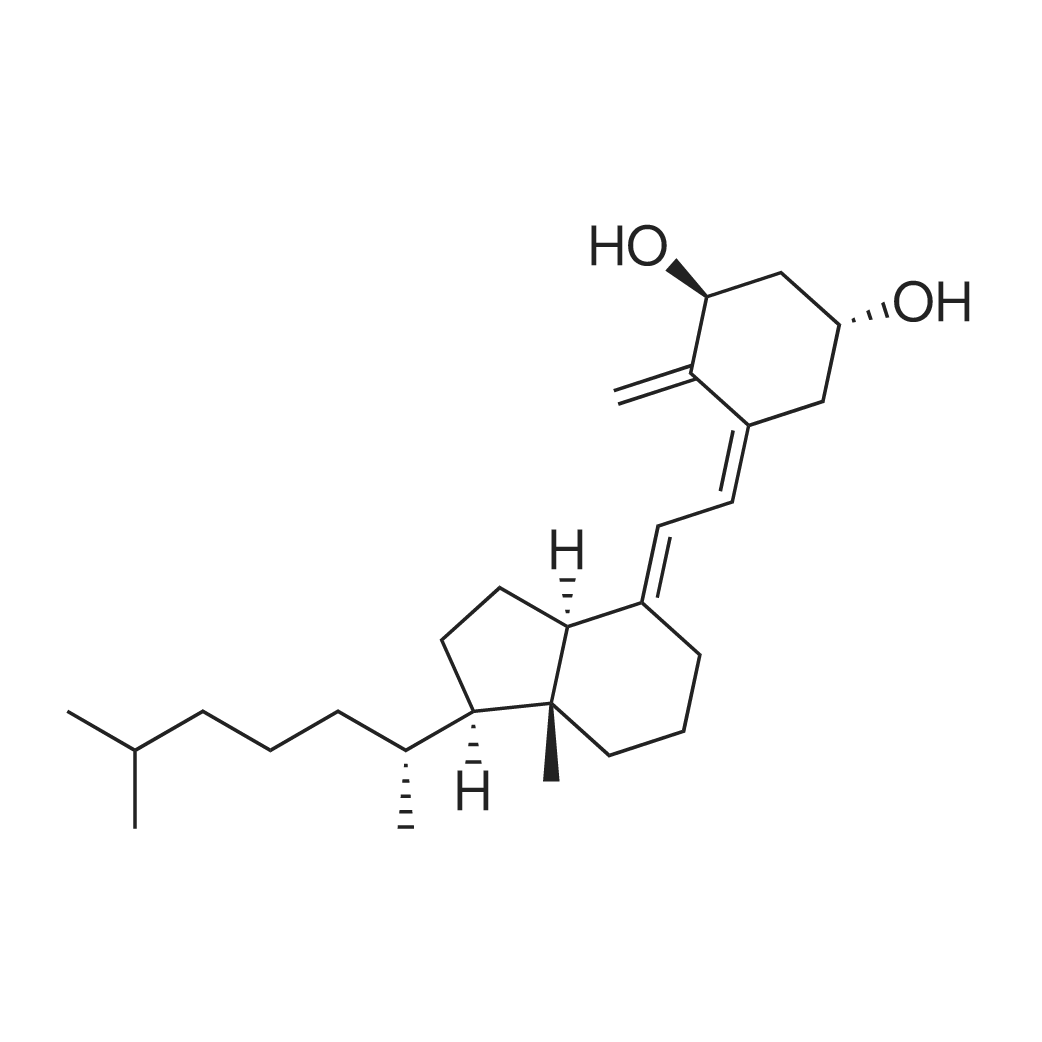
| 描述 | Alfacalcidol is a non-selective VDR activator medication[3]. Alfacalcidol possesses immunoregulatory effects in vitro and in vivo by inhibiting the cytokines IL-1, IL-6, TNF-alpha and particularly IL-12. Therapy with alfacalcidol results in increased production of Th2 helper cells which produce bone protective cytokines like IL-4 and IL-10. Alfacalcidol retards corticosteroid-induced bone loss in contrast to plain vitamin D. Due to its immunomodulating properties, alfacalcidol is particularly suitable for RA(rheumatoid arthritis)-induced bone loss and for the prevention of transplantation osteoporosis, and an adjuvant contribution to the disease-modifying therapy of RA and to the immunosuppressive therapy after transplantation can not be excluded[4]. Alfacalcidol (0.025-0.1 mg/kg; p.o.; five times a week; for 3 months) exerts bone-protective effects independently of its Ca-related effects, and is in this respect superior to vitamin D(3), and that the skeletal actions of alfacalcidol take place, at least in part, independently of suppression of PTH(parathyroid hormone)[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02069886 | Thalassemia (Transfusion Delen... 展开 >>dent) 收起 << | Phase 4 | Withdrawn | December 2018 | - |
| NCT03483441 | Basal Cell Nevus Syndrome ... 展开 >> Basal Cell Carcinoma 收起 << | Phase 1 | Recruiting | May 1, 2023 | United States, Arizona ... 展开 >> University of Arizona College of Medicine Not yet recruiting Phoenix, Arizona, United States, 85004 Contact: Nathalie Zeitouni, MD 602-406-8222 Nathalie.Zeitouni@dignityhealth.org Sub-Investigator: Nathalie Zeitouni, MD United States, Ohio Cleveland Clinic Recruiting Cleveland, Ohio, United States, 44195 Contact: Jeff Negrey, MA 216-636-5504 negreyj2@ccf.org Principal Investigator: Edward Maytin, MD Sub-Investigator: Christine Warren, MD 收起 << |
| NCT02542865 | Growth and Development | Not Applicable | Completed | - | India ... 展开 >> GSK Investigational Site Pune, India, 411001 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.50mL 0.50mL 0.25mL |
12.48mL 2.50mL 1.25mL |
24.96mL 4.99mL 2.50mL |
| 参考文献 |
|---|