| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
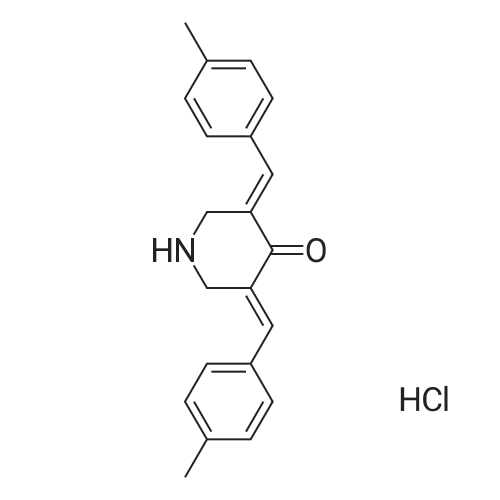
| 描述 | Deubiquitylases (DUBs), isopeptidases that cleave at the carboxyl terminus of ubiquitin, have been divided into five distinct groups, the largest being the ubiquitin-specific proteases (USP/UBP). NSC 632839 is an inhibitor of DUB with IC50 values of 45 nM, 37 nM and 9.8 nM for USP2, USP7, and SENP2, respectively[3]. In vitro, treatment with 10 μM NSC 632839 activated caspase-3/caspase-7 and triggered apoptosis in IMR90-E1A and E1A/C9DN cells. NSC 632839 inhibited IMR90-E1A and E1A/C9DN cells proliferation with IC50 values of 15.65 μM and 16.23 μM[4]. Treatment with 10 μM NSC 632839 strongly induced LC3 puncta formation in Hela cells[5]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.94mL 0.59mL 0.29mL |
14.71mL 2.94mL 1.47mL |
29.42mL 5.88mL 2.94mL |
| 参考文献 |
|---|