| 生物活性 | |||
|---|---|---|---|
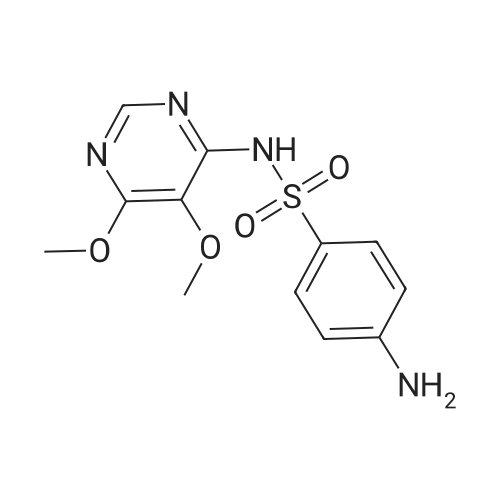
| 描述 | Sulfadoxine is predominantly used in combination with pyrimethamine, commonly known as Fansidar, for the treatment of Plasmodium falciparum. This combination is usually less effective against Plasmodium vivax, probably due to the innate refractoriness of parasites to the sulfadoxine component[2]. Sulfadoxine/pyrimethamine is recommended for intermittent preventative treatment of malaria during pregnancy. During pregnancy, clearance increased 3-fold for sulfadoxine but decreased by 18% for pyrimethamine. Postpartum sulfadoxine clearance decreased gradually over 13 weeks[3]. HIV-infected (HIV(+)) women require more frequent doses of intermittent preventive therapy with SP (Sulfadoxine-pyrimethamine) than do HIV-uninfected (HIV(-)) women. Pregnancy significantly modifies the disposition of SP, whereas HIV status has little influence on pharmacokinetic parameters in pregnant women[4]. SP was associated with a reduced risk of LBW (low birth weight) in HIV-positive women, including those receiving ART, in a low malaria prevalence region[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00852371 | Malaria Inter... 展开 >>mittent Preventive Treatment 收起 << | Phase 3 | Completed | - | - |
| NCT01449045 | Malaria | Phase 3 | Completed | - | Senegal ... 展开 >> University Cheikh Anta Diop Dakar, Senegal 收起 << |
| NCT00941785 | Malaria | Phase 2 Phase 3 | Completed | - | Burkina Faso ... 展开 >> IRSS Bobo-Dioulasso, Burkina Faso, BP545 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.22mL 0.64mL 0.32mL |
16.11mL 3.22mL 1.61mL |
32.22mL 6.44mL 3.22mL |
| 参考文献 |
|---|