| 生物活性 | |||
|---|---|---|---|
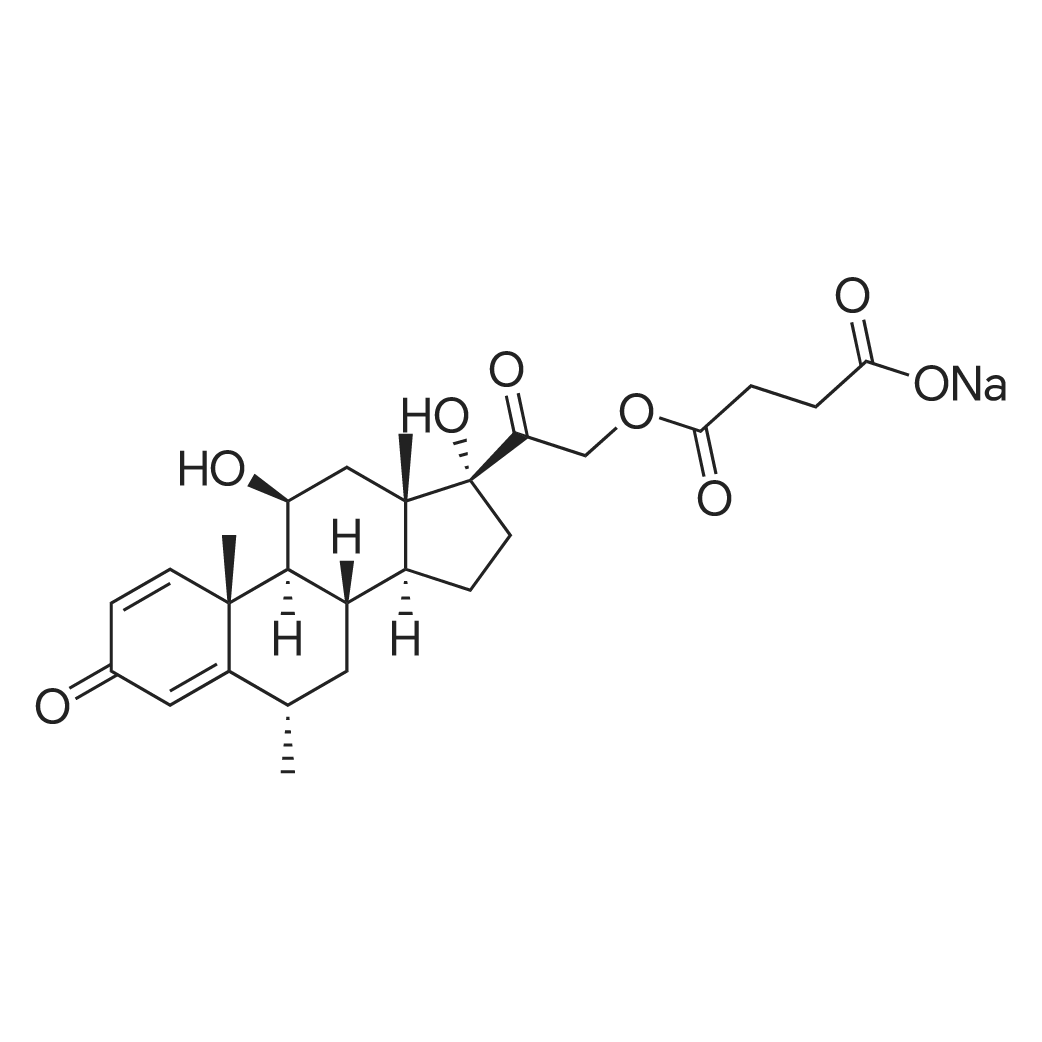
| 描述 | 6α-Methylprednisolone 21-hemisuccinate sodium salt is a glucocorticoid of slightly longer half-life than that of Prednisolone. It has potential uses in anti-inflammatory agents. Methylprednisolone aceponate (MPA) is a non-halogenated corticosteroid with a methyl group at C6, which confers higher intrinsic activity[3]. Methylprednisolone is a synthetic glucocorticoid with a potent and long-acting anti-inflammatory, anti-allergic and immunosuppressant. Methylprednisolone is used in many diseases, such as rheumatic diseases, autoimmune diseases, allergic, anaphylactic shock, asthma. Methylprednisolone was also used in patients with spinal cord injury, in order to minimize neurological damage[4]. There is no difference in efficacy and safety between oral methylprednisolone (oMP) and intravenous methylprednisolone (ivMP). In addition, both routes of administration are equally well tolerated and safe[5]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.01mL 0.40mL 0.20mL |
10.07mL 2.01mL 1.01mL |
20.14mL 4.03mL 2.01mL |
| 参考文献 |
|---|