| 生物活性 | |||
|---|---|---|---|
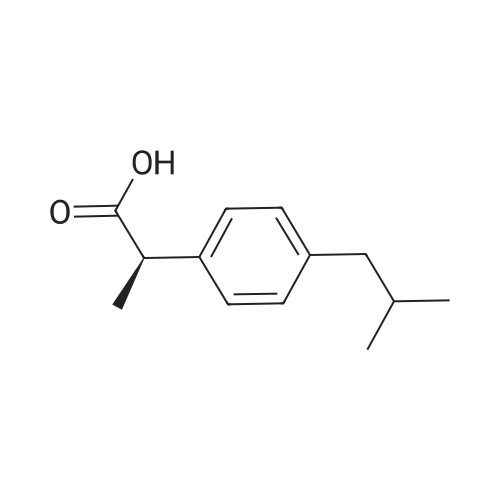
| 描述 | (R)-(-)-Ibuprofen, the R-enantiomer of ibuprofen, does not inhibit cyclooxygenase (COX) enzymes but participates in lipid metabolism pathways and is incorporated into triglycerides alongside endogenous fatty acids[1]. At a concentration of 1 μM, (R)-(-)-Ibuprofen significantly reduces NF-κB activation and fully prevents its induction at 10 μM. However, it inhibits NF-κB luciferase activity with an IC50 of 121.8 μM, which is less effective compared to the S(+)-enantiomer of ibuprofen (IC50 of 61.7 μM). Additionally, (R)-(-)-Ibuprofen at 10 mM does not affect the heat shock factor (HSF)[2]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
4.85mL 0.97mL 0.48mL |
24.24mL 4.85mL 2.42mL |
48.48mL 9.70mL 4.85mL |
| 参考文献 |
|---|