| 生物活性 | |||
|---|---|---|---|
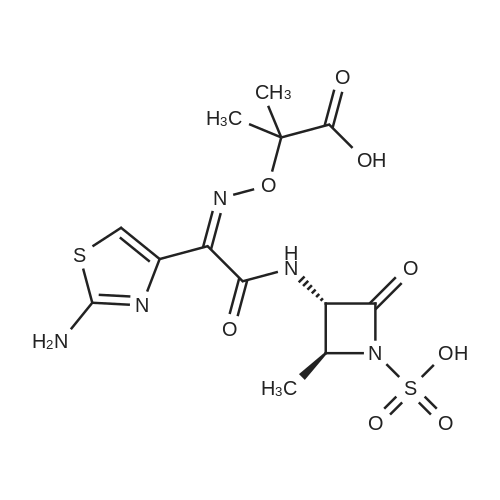
| 描述 | Aztreonam is a new, totally synthetic beta-lactamase agent--the first monobactam. It is highly resistant to hydrolytic inactivation caused by plasmid-mediated (except PSE-2 enzyme found in some Pseudomonas species) or chromosomally mediated beta-lactamases (except for K1 produced by rare strains of Klebsiella oxytoca). The drug exhibits directed antibacterial activity against gram-negative organisms and is effective as monotherapy against most Enterobacteriaceae and Hemophilus and Neisseria species, including beta-lactamase-producing strains; it is not active against anaerobes or gram-positive organisms[3]. In polymicrobial infections or when used for empiric therapy, aztreonam must be combined with other antimicrobial agents active against gram-positive and anaerobic species. Overuse of aztreonam should be avoided to prevent the emergence of resistant P. aeruginosa strains. Except in the treatment of P. aeruginosa infections, aztreonam should not be added to beta-lactam regimens for additional gram-negative[4]. It can be used as a single agent for the treatment of upper urinary tract infections caused by organisms resistant to the cephalosporins and ampicillin. It also can be administered in combination with a drug such as clindamycin for treatment of pelvic inflammatory disease or postoperative pelvic infections[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03440918 | Sepsis Procal... 展开 >>citonin Antimicrobial Stewardship 收起 << | Not Applicable | Recruiting | May 2019 | United States, Tennessee ... 展开 >> Vanderbilt University Medical Center Recruiting Nashville, Tennessee, United States, 37232 收起 << |
| NCT02685930 | - | Completed | - | United States, Missouri ... 展开 >> Barnes-Jewish Hospital Saint Louis, Missouri, United States, 63110 收起 << | |
| NCT03078010 | Intestinal Microbiome ... 展开 >> Febrile Neutropenia 收起 << | Phase 2 | Recruiting | February 2019 | United States, New York ... 展开 >> Memorial Sloan Kettering Cancer Center Recruiting New York, New York, United States, 10065 Contact: Boglarka Gyurkocza, MD 212-639-2860 Contact: Susan Seo, MD 212-639-3151 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.30mL 0.46mL 0.23mL |
11.48mL 2.30mL 1.15mL |
22.97mL 4.59mL 2.30mL |