| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
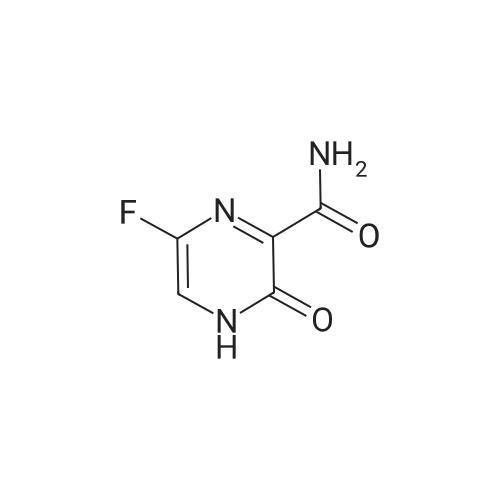
| 描述 | Favipiravir (T 705) selectively inhibits the RNA-dependent RNA polymerase of influenza and other RNA viruses, demonstrating much higher selectivity for viral polymerase over human DNA polymerases and RNA polymerase II[1]. Favipiravir (T 705) functions as a pro-drug, with its cytotoxic effects varying by cell line. It dose-dependently inhibits MNV-induced cytopathic effects (CPE) with an EC50 of 250±11 μM and suppresses MNV RNA synthesis in cell culture with an EC50 of 124±42 μM. Although its antiviral efficacy is relatively modest, Favipiravir can fully stop norovirus replication at 100 μg/mL, a dosage that minimally impacts host cell viability (greater than 80%)[2]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| MDCK cells | Function assay | Inhibition of viral replication of influenza A virus (A/Hong Kong/213/03(H5N1)) and influenza A virus (A/Ann Arbor/6/60(H2N2)) hybrid virus in MDCK cells by neutral red uptake assay | 17194832 | ||
| MDCK cells | Function assay | Inhibition of influenza A virus (A/duck/Minnesota/1525/1981 (H5N1)) replication in MDCK cells by neutral red uptake assay | 17194832 | ||
| Vero cells | Function assay | 7-8 days | Antiviral activity against Junin virus Candid-1 in Vero cells assessed as inhibition of virus-induced visual cytopathic effect after 7 to 8 days | 17606691 | |
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01728753 | Influenza | Phase 1 Phase 2 | Completed | - | United States, Florida ... 展开 >> Miami Research Associates Miami, Florida, United States, 33143 收起 << |
| NCT02662855 | Ebola Virus Disease | Phase 2 | Completed | - | - |
| NCT01068912 | Influenza | Phase 2 | Completed | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
6.37mL 1.27mL 0.64mL |
31.83mL 6.37mL 3.18mL |
63.65mL 12.73mL 6.37mL |
| 参考文献 |
|---|