| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
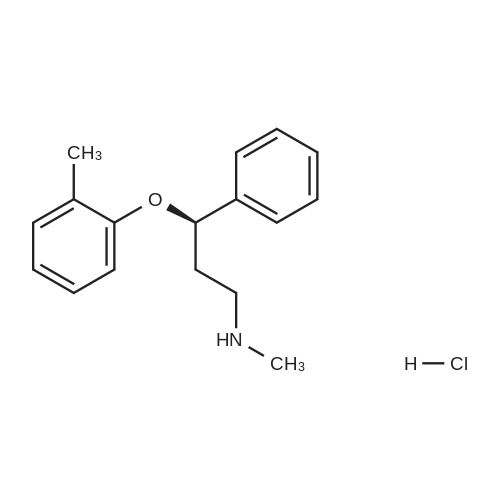
| 描述 | Atomoxetine hydrochloride is a norepinephrine transporter (NET) inhibitor and also acts as an N-methyl-D-aspartate receptor (NMDAR) antagonist. In vivo, atomoxetine (3 mg/kg, intraperitoneal injection) seems to decrease glutamatergic transmission in a brain region-specific manner[3]. ICV (intracerebroventricularly) administration of atomoxetine at 200 – 250 nmol significantly reduced S-IRA (seizure-induced respiratory arrest) evoked by acoustic stimulation in DBA/1 mice. Peripheral atomoxetine administration at a dosage that reduces S-IRA (15 mg/kg, IP) slightly increased basal ventilation and the ventilatory response to 7% CO2[4]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00191880 | Attention Deficit Hyperactivit... 展开 >>y Disorder 收起 << | Phase 3 | Completed | - | Canada ... 展开 >> For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician Calgary, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician Edmonton, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician London, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician Montreal, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician Saskatoon, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician Scarborough, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician St. Johns, Canada For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Monday-Friday from 9:00 AM to 5:00 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician Toronto, Canada 收起 << |
| NCT00286949 | Parkinson's Disease | Not Applicable | Completed | - | United States, Maryland ... 展开 >> Johns Hopkins Hospital Baltimore, Maryland, United States, 21287 收起 << |
| NCT00286949 | - | Completed | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.43mL 0.69mL 0.34mL |
17.13mL 3.43mL 1.71mL |
34.27mL 6.85mL 3.43mL |
| 参考文献 |
|---|