| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
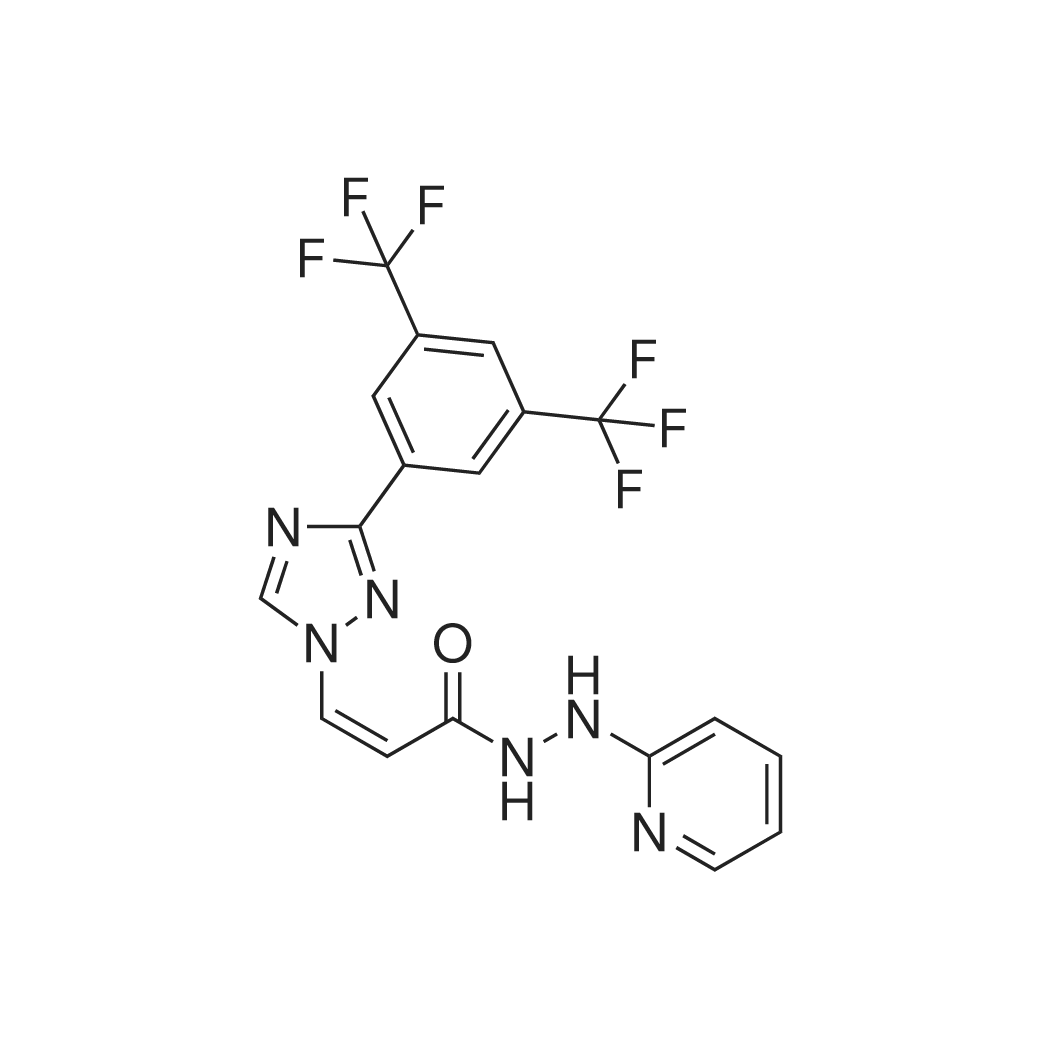
| 描述 | Verdinexor (KPT-335) is an innovative, orally-administered selective inhibitor of nuclear export (SINE) that blocks the nuclear export protein Exportin 1 (XPO1/CRM1), showing efficacy against canine cancer cell lines[1]. KPT-335 effectively hinders cell proliferation, prevents colony formation, and triggers cell apoptosis at doses that are biologically significant. It also leads to a decrease in XPO1 protein levels alongside an increase in XPO1 mRNA levels. Additionally, treatment with KPT-335 elevates the expression and nuclear presence of the tumor suppressor proteins p53 and p21[3]. Prophylactic and therapeutic use of verdinexor offers protection to mice from influenza virus strains A/California/04/09 and A/Philippines/2/82-X79, lowering lung viral counts and inflammatory cytokine levels while showing minimal toxicity[1]. The inhibition of XPO1 by KPT-335 also curtails cyst growth in vivo in the Pkd1 mutant mouse model Pkd1v/v[4]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.26mL 0.45mL 0.23mL |
11.30mL 2.26mL 1.13mL |
22.61mL 4.52mL 2.26mL |
| 参考文献 |
|---|