| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
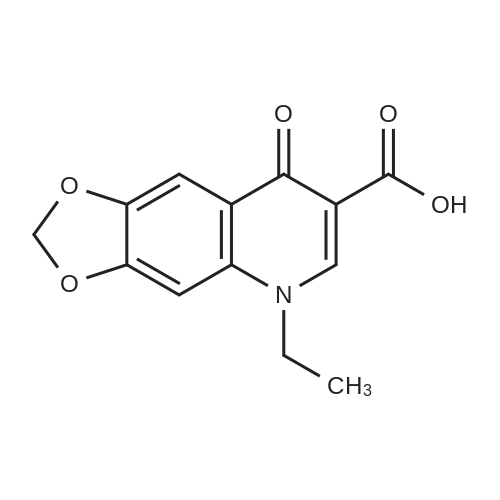
| 描述 | Oxolinic Acid is an antibiotic against both Gram-negative and Gram-positive bacteria. Oxolinic acid can be used for the research of acute and chronic urinary tract infections. Oxolinic acid (2-5 ug/mL) inhibits 115 strains of E. coli. Oxolinic acid (0-31 ug/mL) inhibits 94 % of the 44 strains of Proteus mirabilis. Oxolinic acid (>5 ug/mL) inhibits all strains of Strept. Faecalis[3]. Oxolinic acid reduced RNA synthesis rates whether chromosome supercoiling decreased, increased, or remained unchanged[4]. Oxolinic acid induced benign Leydig cell tumours of the testis in rats at the highest dose level tested (1000 ppm). The no-effect level for tumour induction was confirmed to be 300 ppm (10.9 mg/kg/day) in rats. None was induced in mice[5]. The in vitro minimum inhibitory concentration (MIC) values of oxolinic acid against three strains of Vibrio anguillarum isolated from diseased cod were 0.016 microg mL(-1) (HI-610), 0.250 microg mL(-1) (HI-618) and 0.250 microg mL(-1) (HI-A21)[6]. Oxolinic acid induces Leydig cell tumors in rats by chronically stimulating the release of LH (luteinizing hormone) from the pituitary[7]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.83mL 0.77mL 0.38mL |
19.14mL 3.83mL 1.91mL |
38.28mL 7.66mL 3.83mL |
| 参考文献 |
|---|