| 生物活性 | |||
|---|---|---|---|
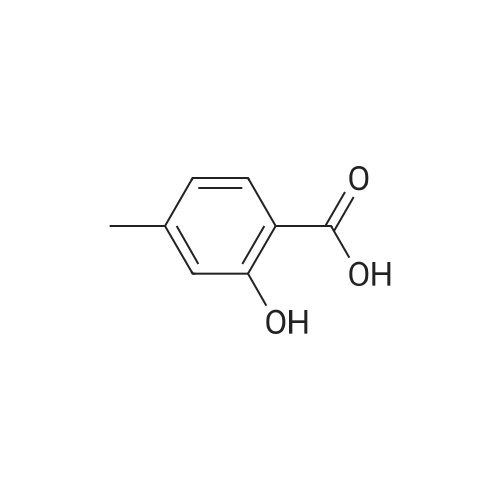
| 描述 | 4-methylsalicylic acid, which do not act as substrates for the medium chain acyl-CoA synthetase, were potent as inhibitors. 4-Methylsalicylic acid was more potent than salicylic acid. The inhibitory carboxylic acids were competitive with respect to hexanoic acid. The distance of the hydroxyl group from the carboxylic acid group of the benzene ring influenced the inhibitory activity. The hydroxyl group on the carbon adjacent to the carboxylic acid group was required for inhibitory activity[2]. A series of 1,3,4-oxadiazole derivatives derived from 4-methoxysalicylic acid or 4-methylsalicylic acid (6a-6z) have been first synthesized for their potential immunosuppressive activity. Among them, compound 6z displayed the most potent biological activity against lymph node cells (inhibition=38.76% for lymph node cells and IC(50)=0.31 μM for PI3Kγ)[3]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
6.57mL 1.31mL 0.66mL |
32.86mL 6.57mL 3.29mL |
65.72mL 13.14mL 6.57mL |
| 参考文献 |
|---|