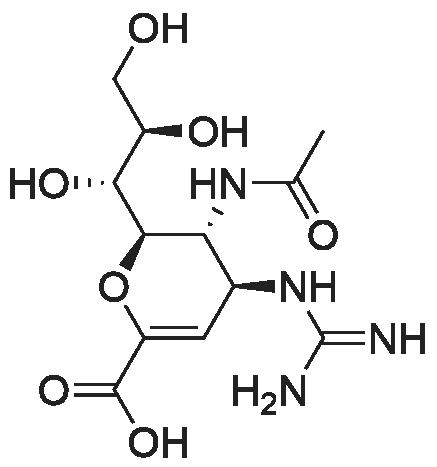
| 生物活性 | |||
|---|---|---|---|
| 描述 | Zanamivir is a potent competitive inhibitor of viral neuraminidase, and inhibits a wide range of influenza A and B types in vitro . The drug is selective for the viral form of the neuraminidase enzyme and does not interact to any significant extent with its human lysosomal equivalent[3]. In Madin-Darby canine kidney (MDCK) cell cultures, IC50 values for zanamivir (i.e. the drug concentration required to inhibit plaque formation of influenza A and B viruses by 50%) in laboratory passaged isolates (0.004 to 0.014 μmol/L) and clinical isolates (0.002 to 16 μmol/L) were largely lower than values for amantadine >25 μmol/L), rimantadine (<0.27 to >25 μmol/L) and ribavirin (6.1 to 54 μmol/L)[4]. Zanamivir was also effective in the inhibition of influenza A [A/Virginia/88 (H3N2) and A/Texas/36/91 (H1N1)] and B (B/Hong Kong/5/72) strains in yield reduction assays in human respiratory epithelium, with concentrations required for 90% inhibition of viral replication (IC90) of <0.03 and 0.75 μmol/L, respectively[5]. In MDCK cell cultures,IC50 values for zanamivir ranged from <0.01 to >100 μmol/L and <0.01 to 21 μmol/L against influenza A strains, from 0.03 to 1.3 μmol/L against influenza B strains[6]. Intranasal zanamivir was 100 to 1000 times more active against influenza A [A/Singapore/1/57(H2N2) and A/Mississippi/1/85 (H3N3)] and B(B/Victoria/102/85) than were amantadine and ribavirin in animal studies. In addition, delayed administration of the drug (for up to 22 hours postinfection) reduced viral replication in ferrets[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00705406 | Acute, Uncomplicated Human Inf... 展开 >>luenza 收起 << | Phase 2 | Completed | - | - |
| NCT00958776 | Cough Sore Th... 展开 >>roat Nasal Congestion Headache Fever Seasonal Influenza 收起 << | Phase 3 | Terminated(This study was term... 展开 >>inated for futility) 收起 << | - | - |
| NCT00705406 | - | Completed | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.01mL 0.60mL 0.30mL |
15.05mL 3.01mL 1.50mL |
30.09mL 6.02mL 3.01mL |
| 参考文献 |
|---|
|
[3] Susan M Cheer ,et al. Zanamivir: an update of its use in influenza. Drugs. 2002;62(1):71-106. |