| 生物活性 | |||
|---|---|---|---|
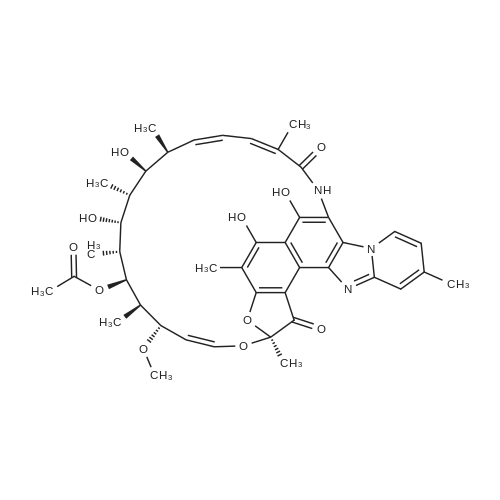
| 描述 | Rifaximin is a broad spectrum oral antibiotic with antimicrobial activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. The use of rifaximin is associated with a low incidence of development, or persistence of spontaneous bacterial mutants[3]. Rifaximin is a broad-range, gastrointestinal-specific antibiotic. Rifaximin may be useful in the treatment of gastrointestinal disorders associated with altered bacterial flora, including irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) [4]. Rifaximin has the potential advantage of preventing bacterial overgrowth and translocation without the systemic side effects of broad-spectrum antibiotics. Rifaximin may be effective in preventing SBP in patients with cirrhosis and ascites compared to systemically absorbed antibiotics and compared to placebo[5]. Rifaximin mitigates ascites and improves survival of cirrhotic patients with refractory ascites[6]. Moreover, combination of rifaximin and lactulose has beneficial effects on HE(hepatic encephalopathy). Compared with lactulose alone, additional rifaximin increases clinical efficacy and decreases mortality[7]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03185611 | Crohn Disease | Phase 3 | Recruiting | July 31, 2018 | China, Guangdong ... 展开 >> The Sixth Affiliated Hospital, Sun Yat-sen University Recruiting Guanzhou, Guangdong, China, 510000 Contact: Xiang Gao, MD, PhD 020-38663423 gaoxiangmed@163.com 收起 << |
| NCT03219528 | Irritable Bowel Syndrome | Phase 4 | Recruiting | February 2020 | United States, Michigan ... 展开 >> University of Michigan Recruiting Ann Arbor, Michigan, United States, 48109 Contact: Deepa Chandhrasekhar Contact: Amy Liu Principal Investigator: Allen Lee 收起 << |
| NCT01662791 | Parkinson's Disease | Phase 3 | Completed | - | United States, Arizona ... 展开 >> Mayo Clinic in Arizona Scottsdale, Arizona, United States, 85259 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.27mL 0.25mL 0.13mL |
6.36mL 1.27mL 0.64mL |
12.72mL 2.54mL 1.27mL |
| 参考文献 |
|---|