| 生物活性 | |||
|---|---|---|---|
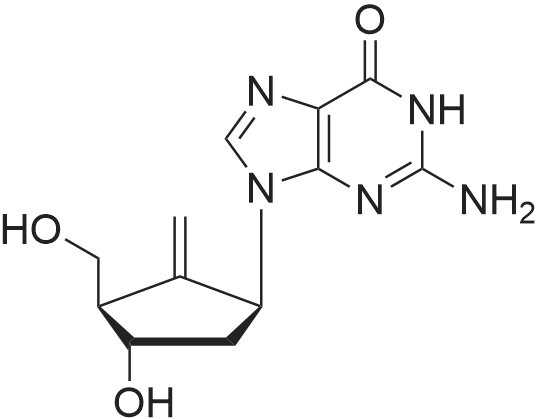
| 描述 | Entecavir( BMS-200475) is a guanosine analogue that inhibits reverse transcription, DNA replication and transcription in the viral replication process, and is a potent, orally-active and selective inhibitor of HBV with an EC50 of 3.75 nM in HepG2 cell.[3]. BMS-200475 has a EC50 of 3.75 nM against HBV. It is incorporated into the protein primer of HBV and subsequently inhibits the priming step of the reverse transcriptase. The antiviral activity of BMS-200475 is significantly less against the other RNA and DNA viruses. Entecavir is more readily phosphorylated to its active metabolites than other deoxyguanosine analogs (penciclovir, ganciclovir, lobucavir, and aciclovir) or lamivudine. The intracellular half-life of entecavir is 15 h[4].BMS-200475-TP and lobucavir-TP competitively inhibit HBV Pol and WHV Pol with respect to the natural dGTP substrate and that both drugs appear to bind to Pol with very high affinities. BMS-200475-TP and lobucavir-TP are nonobligate chain terminators that stall Pol at sites that are distinct yet characteristically two to three residues downstream from dG incorporation sites[5].The concentration of BMS-200475 causing 50% cytotoxicity in 2.2.15 cell cultures was 30 microM, approximately 8,000-fold greater than the concentration required to inhibit HBV replication in the same cell line. Treatment with BMS-200475 resulted in no apparent inhibitory effects on mitochondrial DNA content[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01026610 | Chronic Hepatitis B | Phase 2 | Completed | - | China ... 展开 >> Queen Mary Hospital Hong Kong, China Korea, Republic of Inha University Hospital Incheon, Inchen, Korea, Republic of Hanyang University Guri Hospital Guri, Kyunggi-do, Korea, Republic of Kyungpook National University Hospital Daegu, Korea, Republic of Pusan National University Yangsan Hospital Pusan, Korea, Republic of Kangnam Severance Hospital, Yonsei University Seoul, Korea, Republic of Korea University Medical Center Seoul, Korea, Republic of Severance Hospital of Yonsei University Seoul, Korea, Republic of The Catholic University of Korea, Seoul St. Mary's Hospital Seoul, Korea, Republic of Ulsan University Hospital Ulsan, Korea, Republic of 收起 << |
| NCT01715987 | - | Terminated | - | United States, California ... 展开 >> Asian Pacific Liver Center at St. Vincent Medical Center Los Angeles, California, United States, 90057 United States, New York New Discovery LLC New York, New York, United States, 11355 收起 << | |
| NCT00065507 | Hepatitis B | Phase 3 | Completed | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.61mL 0.72mL 0.36mL |
18.03mL 3.61mL 1.80mL |
36.06mL 7.21mL 3.61mL |
| 参考文献 |
|---|