| 生物活性 | |||
|---|---|---|---|
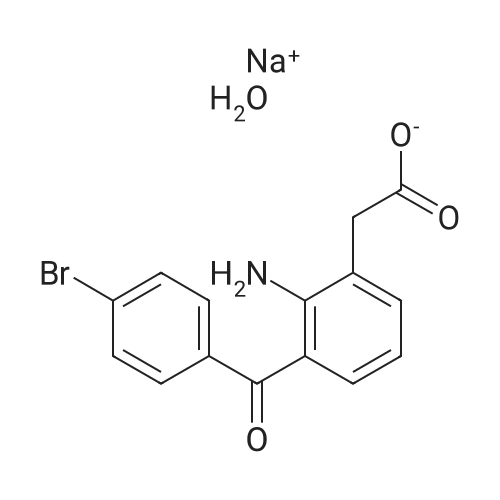
| 描述 | Bromfenac sodium hydrate is a brominated non-steroidal anti-inflammatory/analgesic drug (NSAID), and it is commonly used for the research of postoperative inflammation and pain following cataract surgery, and pseudophakic cystoid macular edema (CME)[2]. Bromfenac (0.0032-3.16%; 100 or 200 μL; rubbed onto the backs) produces significant anti-inflammatory activity at concentrations as low as 0.1% (4 h pretreatment time) or 0.32% (18h pretreatment time) in rats. Bromfenac (0.032-3.16%; 100 μL; rubbed onto the paws) produces dose-related anti-inflammatory activity in rats. Bromfenac (0.32%; 50μL; rubbed onto the abdomen) produces significant blockade of abdominal constriction to ACh challenge in mice[3]. Treatment with 0.1% bromfenac sodium hydrate ophthalmic solution showed good efficacy for preventing cystoid macular edema early after cataract surgery in patients with diabetes[4]. The maintenance of pupil dilation and the prevention of miosis were more effective in the 0.09% bromfenac group than in the control group[5]. Topical bromfenac twice daily may play a role in the reduction of DME (diabetic macular edema) [6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03106402 | - | Completed | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.67mL 0.53mL 0.27mL |
13.36mL 2.67mL 1.34mL |
26.73mL 5.35mL 2.67mL |
| 参考文献 |
|---|