| 生物活性 | |||
|---|---|---|---|
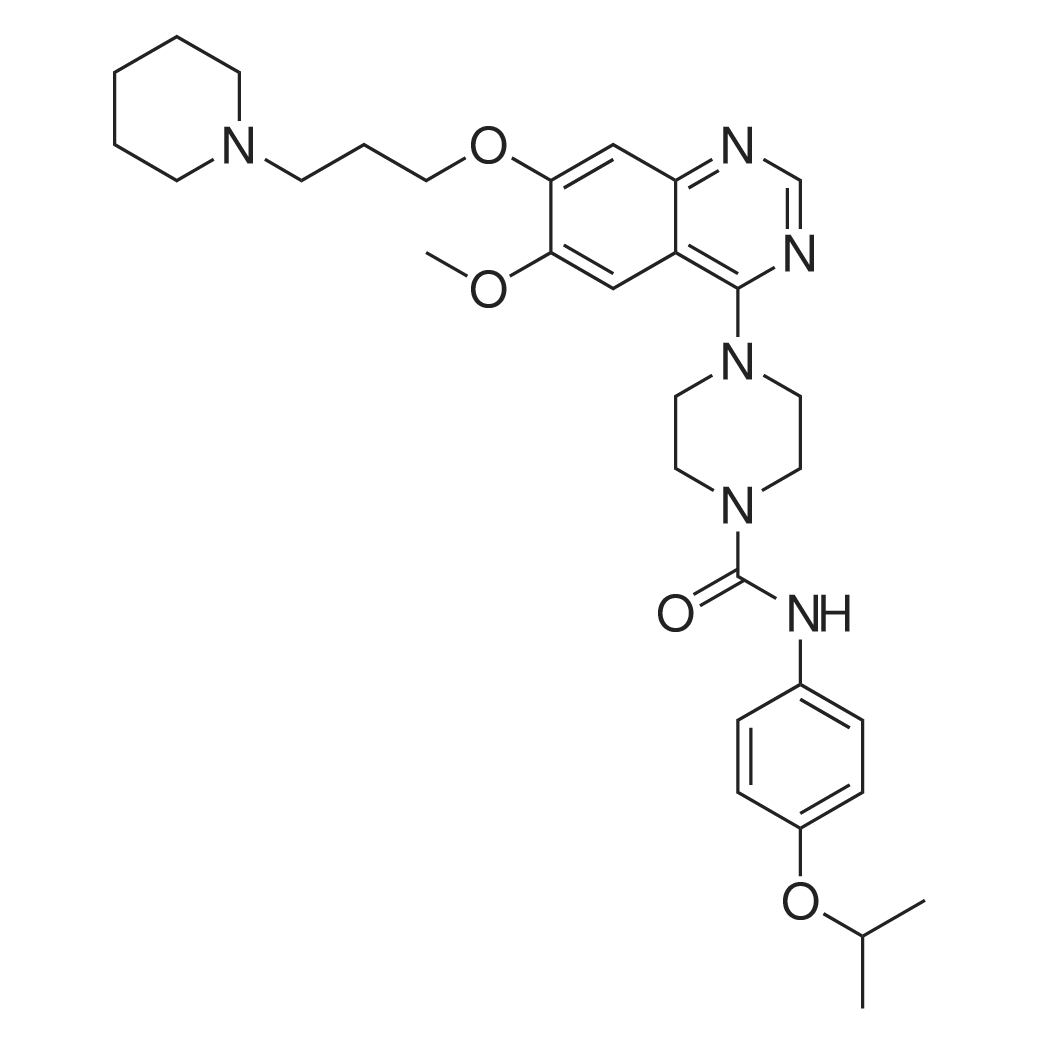
| 描述 | Receptor tyrosine kinases (RTK) are mediators of cellular proliferation. These transmembrane RTKs contain an extracellular domain for ligand binding and intracellular kinase domains that mediate autophosphorylation, recruitment of downstream signaling molecules, and signal transduction. Type III RTKs, such as PDGFR (platelet-derived growth factor receptor), FGFR (fibroblast growth factor receptor), VEGFR (vascular endothelial growth factor receptor), FLT3 and c-KIT are often overexpressed in tumor and implicated in tumor growth and progression. Tandutinib is an inhibitor of FLT3, PDGFR and c-Kit. The kinase inhibitory IC50s were 0.22, 0.20 and 0.17 μM, respectively. Tandutinib inhibited phosphorylation of WT FLT3 and FLT3-ITD (internal tandem duplication) in Ba/F3 cells with IC50s of 30-100nM. Tandutinib inhibited cell proliferation of the FLT3-ITD-positive cells (Molm-13 and Molm-14 cells) with an IC50 value of 10 nM, whereas the FLT3-ITD-negative cells (THP-1, KG-1, and RS4 cells) were resistant, requiring 1000-fold higher concentrations to inhibit cell growth. Treatment of Molm-14 cells with 1 μM tandutinib resulted in an increase of the percentage of apoptotic cells from a background level of 5% to 51% at 24h and 78% at 96h. Tandutinib preferentially inhibited the growth of blast colonies derived from FLT3 ITD-positive patients with IC50s between 75 and 400 nM. In a nude mice model established by injection of FLT3-ITD-transformed Ba/F3 cells, oral administration of tandutinib at 60 mg/kg bid significantly increased the survival of mice and resulted in a significant reduction in mortality in a mouse bone marrow transplantation model. | ||
| 作用机制 | Tandutinib inhibits FLT3, PDGFR and c-Kit in an ATP competitive manner. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00064584 | Acute Myelogenous Leukemia ... 展开 >> Myelodysplastic Syndrome 收起 << | Phase 1 | Completed | - | United States, California ... 展开 >> UCLA Medical Center Los Angeles, California, United States, 90095 United States, Massachusetts Dana Farber Cancer Institute Boston, Massachusetts, United States, 02115 United States, New York Memorial Sloan Kettering Cancer Center New York, New York, United States, 10021 United States, Ohio Ohio State University Medical Center Columbus, Ohio, United States, 43210 United States, Oregon Oregon Health Sciences University Portland, Oregon, United States, 97201 收起 << |
| NCT00274248 | Acute Myelogenous Leukemia | Phase 1 | Completed | - | United States, Massachusetts ... 展开 >> The Dana Farber Cancer Institute Boston, Massachusetts, United States, 02134 收起 << |
| NCT00297921 | Acute Myelogenous Leukemia | Phase 1 Phase 2 | Withdrawn | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.78mL 0.36mL 0.18mL |
8.89mL 1.78mL 0.89mL |
17.77mL 3.55mL 1.78mL |
| 参考文献 |
|---|