| 生物活性 | |||
|---|---|---|---|
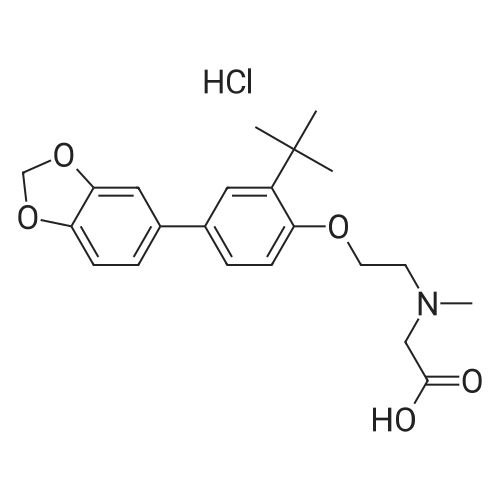
| 描述 | The glycine transporter 1 (GlyT1) is a target for the treatment of central nervous system disorders related to hypoglutamatergic function. LY2365109 is a selective GlyT1 inhibitor that inhibits [14C] glycine uptake in cells transfected with hGlyT1a with an IC50 value of 15.8 nM. The administration of rats with LY2365109 (0.3 – 30 mg/kg, p.o.) for 1 h led to a dose-dependent elevation in the cerebrospinal fluid level of glycine. LY2365109 at a dose of 3 mg/kg significantly increased NMDA-induced release of dopamine at 1.5h post-dosing relative to the controls. LY2365109 at 10 mg/kg significantly enhanced dopamine release at 1.5 - 2h after treatment. Rats treated with LY2365109 (3 and 10 mg/kg, p.o.) also showed impaired gait with ataxic like behavior and compulsive “toe walking” around the cage at 8h after LY2365109 administration[3]. | ||
| 作用机制 | LY2365109 is a sarcosine-like GlyT1 inhibitor that potentiate NMDA function in the prefrontal cortex and mediates sustained inhibition of GlyT1 transporter in the caudal areas of the brain[3]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.37mL 0.47mL 0.24mL |
11.85mL 2.37mL 1.19mL |
23.70mL 4.74mL 2.37mL |
| 参考文献 |
|---|