| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
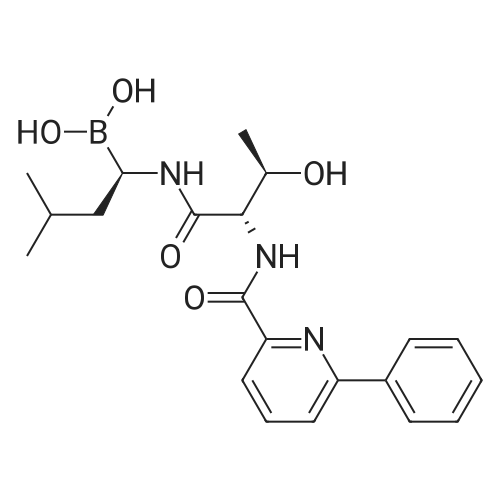
| 描述 | The ubiquitin-proteasome pathway plays a key role in protein processing and degradation, regulating crucial transduction pathways for cell growth and survival, including cell-cycle control, transcriptional regulation, cellular stress responses, and antigen presentation. Delanzomib (CEP-18770) is an orally active proteasome inhibitor that has potency against chymotrypsin-like proteasome activity with IC50 value of 3.8 nM[2]. In vitro, treatment with 20 nM delanzomib suppressed nuclesar factor-κB (NF-κB) mediated transcription and induced apotosis in multiple myeloma cell lines CMA-03, H929, KMM1, KMS11, KMS18, KMS27, SKMM1, U266 and RPMI-8226. Delanzomib can inhibit cell proliferation, including A2780, PC3, H460 SC, LoVo, RPMI8226 and HS-Sultan cell lines, with IC50 values of 13.7 nM, 22.2 nM, 34.2 nM, 11.3 nM, 5.6 nM and 8.2 nM, respectively. These data indicated that delanzomib exhibits a favorable cytotoxicity profile toward normal human epithelial cells, bone marrow progenitors, and bone marrow–derived stromal cells[2]. In vivo, delanzomib demonstrated dose-related inhibition of tumor proteasome activity in RPMI-8266 tumor-bearing SCID mice. Oral administration of delanzomib at 10 mg/kg twice a week resulted in a significant reduction of tumor weight and oral administration of delanzomib at 13 mg/kg resulted in complete tumor regression in RPMI-8226 subcutaneous xenograft SCID mice modelPiva R, Ruggeri B, Williams M, et al. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood, 2008, 111(5): 2765-2775.|https://www.ncbi.nlm.nih.gov/pubmed/18057228}. Treatment with delanzomib either 3 mg/kg once or twice weekly intravenously or orally at 10 mg/kg suppressed the development and gression of renal tissue renal tissue damage and extended the survival of the NZBWF1 mice with fatal lupus nephritis{{Seavey M M, Lu L D, Stump K L, et al. Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. International Immunopharmacology, 2012, 12(1): 257-270.|http://www.ncbi.nlm.nih.gov/pubmed/22178195. | ||
| 作用机制 | Delanzomib reversible inhibits P2 threonine boronic acid of the 26S mammalian proteasome[3]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00572637 | Solid Tumors ... 展开 >>Lymphoma, Non-Hodgkin 收起 << | Phase 1 | Completed | - | Italy ... 展开 >> Europen Institute of Oncology Milano, Italy, 20141 Switzerland IOSI - Oncology Institute of Southern Switzerland - Ospedale S. Giovanni Bellinzona, Switzerland, 6500 Kantonsspital St. Gallen St. Gallen, Switzerland, 9007 收起 << |
| NCT01348919 | Multiple Myeloma | Phase 1 Phase 2 | Terminated | - | United States, Georgia ... 展开 >> Teva Investigational Site 1 Augusta, Georgia, United States United States, Kentucky Teva Investigational Site 3 Lexington, Kentucky, United States United States, Texas Teva Investigational Site 2 Houston, Texas, United States New Zealand Teva Investigational Site 201 Auckland, New Zealand Teva Investigational Site 204 Auckland, New Zealand Teva Investigational Site 200 Christchurch, New Zealand Teva Investigational Site 206 Hamilton, New Zealand Teva Investigational Site 205 Newtown, New Zealand Teva Investigational Site 202 Palmerston North, New Zealand Teva Investigational Site 203 Takapuna, New Zealand 收起 << |
| NCT01023880 | Multiple Myeloma | Phase 1 Phase 2 | Terminated | - | United States, Arizona ... 展开 >> Mayo Clinic- Scottsdale Scottsdale, Arizona, United States United States, Arkansas University of Arkansas for Medical Sciences Little Rock, Arkansas, United States United States, California Stanford Heme Group Palo Alto, California, United States University of California, San Francisco San Francisco, California, United States United States, District of Columbia Washington Cancer Institute Washington, District of Columbia, United States United States, Illinois Northwestern University Medical School Chicago, Illinois, United States United States, Michigan Henry Ford Health System Protocol Review Committee Detroit, Michigan, United States Sparrow Regional Cancer Center Lansing, Michigan, United States United States, Missouri Washington University School of Medicine St. Louis, Missouri, United States United States, New Jersey John Theurer Cancer Center Hackensack, New Jersey, United States United States, North Carolina Duke University Medical Center Durham, North Carolina, United States United States, Pennsylvania University of Pennsylvania Philadelphia, Pennsylvania, United States United States, Wisconsin Medical College of Wisconsin Milwaukee, Wisconsin, United States 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.42mL 0.48mL 0.24mL |
12.10mL 2.42mL 1.21mL |
24.20mL 4.84mL 2.42mL |
| 参考文献 |
|---|