| 生物活性 | |||
|---|---|---|---|
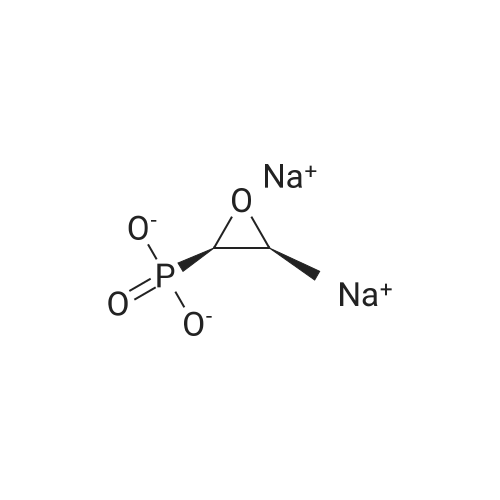
| 描述 | Fosfomycin (disodium salt) is a bactericidal, low-molecular weight, broad-spectrum antibiotic, with putative activity against several bacteria, including multidrug-resistant Gram-negative bacteria, by irreversibly inhibiting an early stage in cell wall synthesis. Fosfomycin has a synergistic effect when used in combination with other antimicrobial agents that act via a different mechanism of action, thereby allowing for reduced dosages and lower toxicity[3]. Oral fosfomycin is mainly used in the treatment of urinary tract infections, particularly those caused by Escherichia coli and Enterococcus faecalis. Intravenous fosfomycin has been administered in combination with other antibiotics for the treatment of nosocomial infections due to multidrug-resistant (MDR) Gram-positive and Gram-negative bacteria. Fosfomycin is well tolerated, with a low incidence of adverse events[4]. Fosfomycin is the treatment of choice for cystitis in immunocompetent patients, patients with transplants, pregnant women and in pediatric settings. The drug is especially useful due to its microbiological activity and oral posology in cystitis caused by ESBL bacteria. Administer intravenously at high doses and combined with other antimicrobial agents, Fosfomycin has also been shown active in combination with daptomycin or imipenem in osteoarticular infections by methicillin-resistant Staphylococcus aureus[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02178254 | Pseudomonas Infection | Phase 1 | Completed | - | United States, Kansas ... 展开 >> Quintiles Phase I Services - Overland Park Overland Park, Kansas, United States, 66211-1553 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
5.49mL 1.10mL 0.55mL |
27.47mL 5.49mL 2.75mL |
54.94mL 10.99mL 5.49mL |
| 参考文献 |
|---|