| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
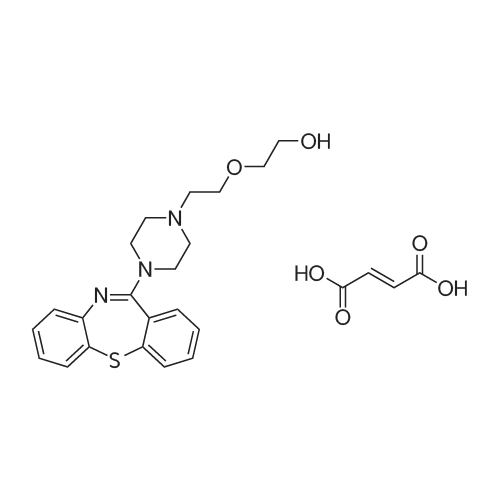
| 描述 | Quetiapine fumarate, as a 5-HT receptors agonist and a dopamine receptor antagonist, has antidepressant and anxiolytic effects[3]. Quetiapine resulted in significantly greater cognitive impairment, higher rates of falls and injury and increased mortality in patients with parkinsonism, but not in patients with dementia. Compared with risperidone and olanzapine, quetiapine had significantly lower risk of mortality, reduced rate of cerebrovascular events, increased rate of falls and injury and less metabolic disorders compared with olanzapine, but higher metabolic disorders compared with risperidone[4]. Quetiapine monotherapy is effective for acute bipolar depression and the prevention of mania/hypomania switching. Its common adverse effects are extrapyramidal side effects, sedation, somnolence, dizziness, fatigue, constipation, dry mouth, increased appetite, and weight gain[5]. Based on this meta-analysis, quetiapine-XR (extended-release) is efficacious in the treatment of GAD (generalized anxiety disorder) in adult patients. Despite its low acceptability and tolerability, the use of 50-150 mg/day quetiapine-XR for adult GAD patients may be considered as an alternative treatment[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00393978 | Bipolar Disorder ... 展开 >> Cannabis-Related Disorder 收起 << | Phase 4 | Completed | - | United States, Ohio ... 展开 >> University of Cincinnati Medical Center Cincinnati, Ohio, United States, 45219-0516 收起 << |
| NCT00393978 | - | Completed | - | - | |
| NCT00306540 | Post-Traumatic Stress Disorder | Phase 3 | Completed | - | Australia, Queensland ... 展开 >> Research Site Brisbane, Queensland, Australia Australia, South Australia Research Site Adelaide, South Australia, Australia Australia, Victoria Research Site Melbourne, Victoria, Australia 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.13mL 0.23mL 0.11mL |
5.66mL 1.13mL 0.57mL |
11.32mL 2.26mL 1.13mL |
| 参考文献 |
|---|