| 生物活性 | |||
|---|---|---|---|
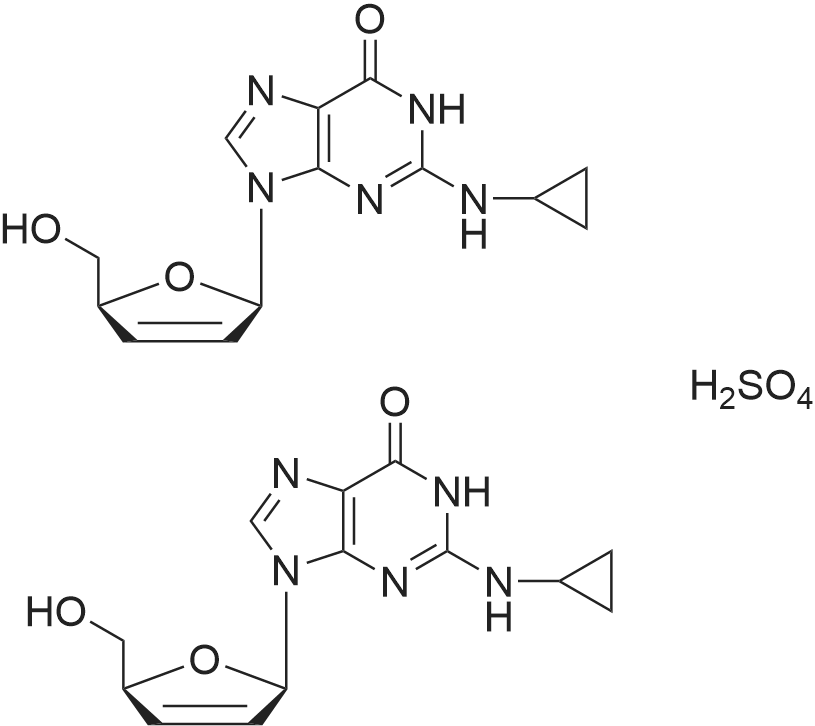
| 描述 | Abacavir Hemisulfate (ABC Sulfate) is a competitive and brain-penetrant reverse transcriptase inhibitor. Absolute bioavailability of oral abacavir hemisulfate was 83% and can be administered with or without meals[3]. Abacavir has been approved for treatment of HIV infection in combination with other anti-HIV agents. About 70% of patients receiving abacavir (300 mg b.i.d) had less than 400 copies/mL of HIV RNA at 16 weeks[4]. HIV-infected patients received therapy switch from tenofovir disoproxil fumatate and emtricitabine (TDF/FTC) to abacavir and lamivudine (ABC/3TC), after 3 months, the low-density lipoprotein (LDL), high-density lipoprotein (HDL), and total cholesterol (TC) levels increased by a median (interquartile range) of 17 (7, 32), 6 (2, 13), and 27 (10, 45) mg/dL, respectively[5]. Recently, Abacavir Hemisulfate showed effect on novel pneumonia caused by 2019-novel coronavirus. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00085943 | HIV Infection ... 展开 >> Infection, Human Immunodeficiency Virus 收起 << | Phase 3 | Completed | - | - |
| NCT00002409 | HIV Infections | Phase 3 | Completed | - | - |
| NCT02342769 | - | Active, not recruiting | October 15, 2018 | Germany ... 展开 >> GSK Investigational Site Freiburg, Baden-Wuerttemberg, Germany, 79106 GSK Investigational Site Mannheim, Baden-Wuerttemberg, Germany, 68161 GSK Investigational Site Stuttgart, Baden-Wuerttemberg, Germany, 70197 GSK Investigational Site Ulm, Baden-Wuerttemberg, Germany, 89081 GSK Investigational Site Muenchen, Bayern, Germany, 80331 GSK Investigational Site Muenchen, Bayern, Germany, 80336 GSK Investigational Site Muenchen, Bayern, Germany, 80801 GSK Investigational Site Frankfurt am Main, Hessen, Germany, 60590 GSK Investigational Site Frankfurt, Hessen, Germany, 60596 GSK Investigational Site Osnabrueck, Niedersachsen, Germany, 49090 GSK Investigational Site Aachen, Nordrhein-Westfalen, Germany, 52062 GSK Investigational Site Duesseldorf, Nordrhein-Westfalen, Germany, 40237 GSK Investigational Site Koeln, Nordrhein-Westfalen, Germany, 50674 GSK Investigational Site Mainz, Rheinland-Pfalz, Germany, 55116 GSK Investigational Site Magdeburg, Sachsen-Anhalt, Germany, 39120 GSK Investigational Site Berlin, Germany, 10243 GSK Investigational Site Berlin, Germany, 10405 GSK Investigational Site Berlin, Germany, 10707 GSK Investigational Site Berlin, Germany, 10777 GSK Investigational Site Berlin, Germany, 10961 GSK Investigational Site Berlin, Germany, 13347 GSK Investigational Site Berlin, Germany, 14057 GSK Investigational Site Chemnitz, Germany, 09111 GSK Investigational Site Dortmund, Germany, 44137 GSK Investigational Site Hamburg, Germany, 20099 GSK Investigational Site Hamburg, Germany, 20246 GSK Investigational Site Koeln, Germany, 50668 GSK Investigational Site Koeln, Germany, 50679 GSK Investigational Site Weimar, Germany, 99427 收起 << | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.49mL 0.30mL 0.15mL |
7.45mL 1.49mL 0.75mL |
14.91mL 2.98mL 1.49mL |
| 参考文献 |
|---|