产品说明书
| 生物活性 | |||
|---|---|---|---|
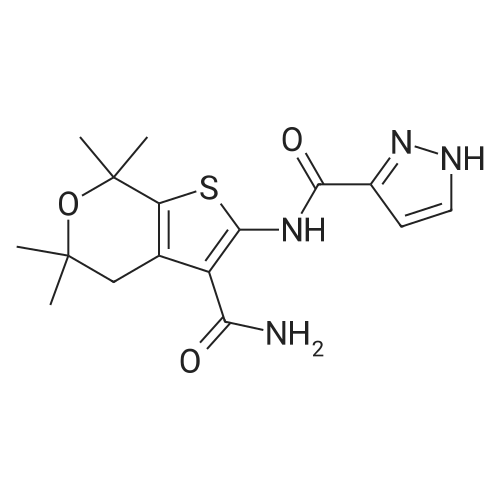
| 描述 | Cystic fibrosis (CF) is the most common life-threatening autosomal recessive disorder in Caucasian populations. It is caused by mutations of the gene for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The most common mutation, F508del, belongs to the class II trafficking defects, where folding of the CFTR protein is impaired, resulting in a reduction of the amount of ion channel present on the cell surface. With the G551D mutation (class III), the amount of protein is not affected but its open probability is reduced, resulting as well in a reduced channel gating[2]. GLPG1837 is a potent and reversible CFTR potentiator, with EC50s of 3 nM and 339 nM for F508del and G551D CFTR, respectively[2]. GLPG1837 had an attractive in vitro ADME (ADME: absorption, distribution, metabolism, excretion) profile, showing low Clint, unb in both microsomal and hepatocytes stability assays, good permeability, and no off-target inhibition of CYPs and the hERG channel[2]. GLPG1837 is reversible CFTR potentiator, with an apparent affinity within a range of 0.2 ∼ 2 µM[3]. The pharmacokinetic profile of GLPG1837 was attractive, showing a low Cl, unb and good F% in both rat and dog. In rats and dogs, the clearance rate (CL) of 1 mg/kg GLPG1837 was 1.92 and 0.32 L/h/kg, respectively. In addition, T1/2 was 1.84 hours in rats while 3 hours in dogs. The oral bioavailability of GLPG1837 was 67% in rats and more than 100% in dogs with oral administration of 5 mg/kg[2]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02325037 | Healthy | Phase 1 | Completed | - | Belgium ... 展开 >> SGS LSS Clinical Pharmacology Unit Antwerp Antwerp, Belgium 收起 << |
| NCT02562950 | Healthy | Phase 1 | Completed | - | Belgium ... 展开 >> SGS LSS Clinical Pharmacology Unit Antwerp Antwerp, Belgium 收起 << |
| NCT02707562 | Cystic Fibrosis | Phase 2 | Completed | - | Australia ... 展开 >> Royal Adelaide Hospital Adelaide, Australia The Prince Charles Hospital Chermside, Australia Monash Medical Centre Clayton, Australia Sir Charles Gairdner Hospital Nedlands, Australia Mater Adult Hospital South Brisbane, Australia Czech Republic Fakultni nemocnice v Motole Praha 5, Czech Republic Germany Charité Universitätsmedizin Berlin Berlin, Germany Universitätsklinkikum Koeln Cologne, Germany Uniklinik Carl-Gustav-Carus Dresden, Germany Lungenheilkunde München-Pasing München, Germany Ireland Beamont Hospital Dublin, Ireland St. Vincent's University Hospital Dublin, Ireland United Kingdom Queen Elizabeth University Hospital Glasgow, United Kingdom Liverpool Heart and Chest Hospital Liverpool, United Kingdom Royal Brompton Hospital London, United Kingdom The Medicines Evaluation Unit Ltd Manchester, United Kingdom 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.87mL 0.57mL 0.29mL |
14.35mL 2.87mL 1.44mL |
28.70mL 5.74mL 2.87mL |
| 参考文献 |
|---|