| 生物活性 | |||
|---|---|---|---|
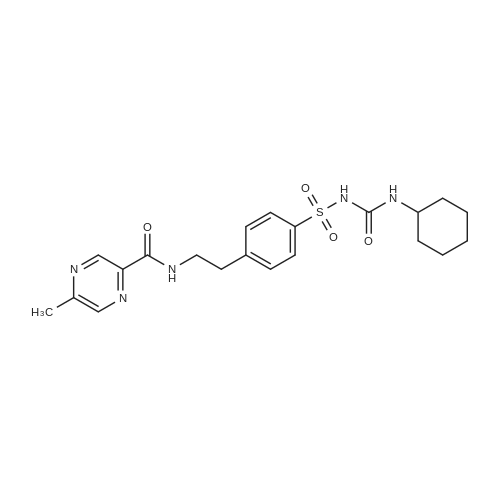
| 描述 | Glipizide is used to treat high blood sugar levels caused by a type of diabetes mellitus called type 2 diabetes. It is classified as a second generation sulfonylurea, which means that it undergoes enterohepatic circulation. Mechanism of action is produced by blocking potassium channels in the beta cells of the islets of Langerhans. By partially blocking the potassium channels, the cell remains depolarized, increasing the time the cell spends in the calcium release stage, which results in signaling leading to calcium influx. The increase in calcium will initiate more insulin release from each beta cell. Sulfonylureas may also cause the decrease of serum glucagon and potentiate the action of insulin at the extrapancreatic tissues[3]. Glipizide treatment decreased dilated renal tubule number, improved glomerulus integrity, and reduced inflammatory infiltration. Glipizide blocks renal interstitial fibrosis by inhibiting AKT signaling pathway[4]. Glipizide release from mucoadhesive microcapsules was slow and extended over longer periods of time and depended on composition of the coat. In the in vivo evaluation, alginate-Carbopol microcapsules could sustain the hypoglycemic effect of glipizide over a 14-hour period[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02456428 | - | Completed | - | Canada, Quebec ... 展开 >> Lady Davis Institute for Medical Research, Jewish General Hospital Montreal, Quebec, Canada, H3T1E2 收起 << | |
| NCT02475499 | - | Completed | - | Canada, Quebec ... 展开 >> Lady Davis Institute for Medical Research, Jewish General Hospital Montreal, Quebec, Canada, H3T1E2 收起 << | |
| NCT03492580 | - | Completed | - | United States, New Jersey ... 展开 >> Janssen Investigative Site Titusville, New Jersey, United States, 08560 收起 << | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.24mL 0.45mL 0.22mL |
11.22mL 2.24mL 1.12mL |
22.44mL 4.49mL 2.24mL |
| 参考文献 |
|---|