| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
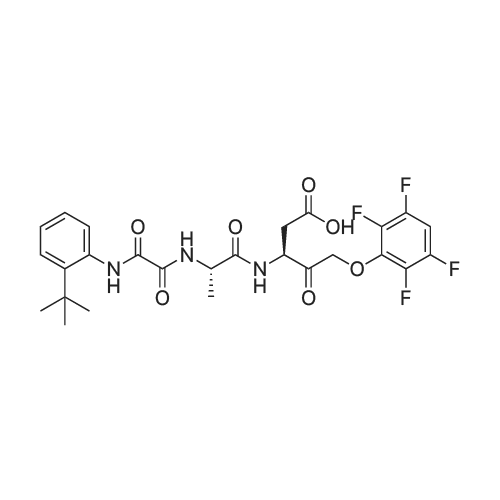
| 描述 | Caspases are a group of enzymes that function as executors of apoptosis. Emricasan is a pan-caspase inhibitor with IC50 values of 0.13 – 0.9μM for SNB-19 cells against three Zika virus strains in both caspase activity and cell viability assays. Emricasan at a concentration of 15 or 30μM reduced the number of cleaved caspase-3-expressing forebrain-specific human neural progenitor cells infected by FSS13025 (a Zika virus strain) in both monolayer and 3D organoid cultures.[2] In C57BL/6J mice fed a high-fat diet (HFD), treatment with emricasan (0.3mg/kg/day, i.g.) for 20 weeks reduced the amount of TUNEL-positive cells and decreased the levels of caspase-3 and caspase-8 in the liver compared with the non-emricasan-treated group. Emricasan treatment also decreased HFD-induced upregulation of AST and ALT in the serum. The expression of inflammatory mediators, including TNF-α, IL-1β, MCP-1, and CXCL2 in HFD fed mice was significantly suppressed by emricasan treatment. Emricasan-treated mice also showed reduced transcripts for α-SMA and collagen-1α, as well as less fibrosis compared to HFD-fed mice without emricasan treatment. [1] | ||
| 作用机制 | Emricasan is an irreversible, orally active pan-caspase inhibitor.[1] | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| JFas cells | Function assay | Inhibitory concentration against JFas cells, IC50=0.025 μM | 16250635 | ||
| THP-1 cells | Function assay | Inhibitory concentration against THP-1 cells, IC50=0.27 μM | 16250635 | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00088140 | Hepatitis C, Chronic | Phase 2 | Completed | - | United States, Arizona ... 展开 >> Mayo Clinic Hospital Phoenix, Arizona, United States, 85054 United States, California Scripps Clinic La Jolla, California, United States, 92067 California Pacific Medical Center San Francisco, California, United States, 94115 University of California, San Francisco San Francisco, California, United States, 94143 United States, Florida University of Miami Miami, Florida, United States, 33136 United States, Indiana Indiana University School of Medicine Indianapolis, Indiana, United States, 46202 United States, Massachusetts Beth Israel Deaconness Medical Center Boston, Massachusetts, United States, 02215 United States, Michigan Henry Ford Hospital Detroit, Michigan, United States, 48202 United States, Minnesota The Mayo Clinic Rochester, Minnesota, United States, 55905 United States, New York Mt. Sinai School of Medicine New York City, New York, United States, 10029 United States, North Carolina University of North Carolina Chapel Hill, North Carolina, United States, 27599 Duke University Medical Center Durham, North Carolina, United States, 27715 United States, Ohio University of Cincinnati Cincinnati, Ohio, United States, 45267 Metrohealth Medical Center Cleveland, Ohio, United States, 44109 United States, Virginia Medical College of Virginia Richmond, Virginia, United States, 23298 收起 << |
| NCT00080236 | Liver Transplantation ... 展开 >> Hepatitis Cholestasis Carcinoma, Hepatocellular 收起 << | Phase 2 | Completed | - | United States, Arizona ... 展开 >> Mayo Clinic Scottsdale Phoenix, Arizona, United States, 85054 United States, California University of California Los Angeles Los Angeles, California, United States, 90095 University of California San Francisco San Francisco, California, United States, 94143 United States, Indiana Indiana University Medical Center Indianapolis, Indiana, United States, 46202 United States, Louisiana Tulane University Hospital and Clinic New Orleans, Louisiana, United States, 70112 United States, Minnesota Mayo Clinic Rochester Rochester, Minnesota, United States, 55905 United States, New York Mount Sinai School of Medicine New York City, New York, United States, 10029 United States, Ohio University of Cincinnati Cincinnati, Ohio, United States, 45267 United States, Texas Baylor Regional Transplant Institute, Baylor University Medical Center Dallas, Texas, United States, 75246 The University of Texas Health Science Center at San Antonio San Antonio, Texas, United States, 78229 Germany Oberarzt der Klinik für Allgemein-, Viszeral- und Transplantationschirurgie Charité-Virchow Berlin, Germany, D-13353 Klinik für Viszeral- und Transplantationschirurgie Medizinische Hochschule Hannover Hannover, Germany, D-30623 Abteilung für Transplantationschirurgie Johannes Gutenberg-Universität Mainz Mainz, Germany, 55101 收起 << |
| NCT01937130 | Acute on Chronic Hepatic Failu... 展开 >>re Acute Liver Failure Liver Cirrhosis Acute Alcoholic Hepatitis 收起 << | Phase 2 | Terminated(Data needs met) | - | United States, Alabama ... 展开 >> University of Alabama at Birmingham Birmingham, Alabama, United States, 35294 United States, California Scripps Clinic La Jolla, California, United States, 92037 VA San Diego Healthcare System San Diego, California, United States, 92161 Sutter Pacific Medical Foundation San Francisco, California, United States, 94115 United States, District of Columbia Georgetown University Hospital Washington, District of Columbia, United States, 20007 United States, Kansas University of Kansas Medical Center Kansas City, Kansas, United States, 66160 United States, Kentucky Univerisity of Louisville Liver Research Center Louisville, Kentucky, United States, 40202 United States, Mississippi University of Mississippi Medical Center Jackson, Mississippi, United States, 39216 United States, New York Montefiore Medical Center Bronx, New York, United States, 10467 United States, Utah University of Utah Salt Lake City, Utah, United States, 84132 United States, Washington University of Washington Harborview Medical Center Seattle, Washington, United States, 98104 United Kingdom Singleton Hospital Swansea, Wales, United Kingdom, SA2 8QA Basildon and Thurrock University Hospital Basildon, United Kingdom, SS16 5NL Blackpool Victoria Hospital Blackpool, United Kingdom, FY3 8NR Bristol Royal Infirmary Bristol, United Kingdom, BS2 8HW Ninewells Hospital Dundee, United Kingdom, DD1 9SY Glasgow Royal Infirmary Glasgow, United Kingdom Leicester Royal Infirmary Leicester, United Kingdom, LE1 5WW Royal Liverpool University Hospital Liverpool, United Kingdom, L7 8XP University College London, Royal Free Hospital London, United Kingdom, NW3 2PF Royal London Hospital London, United Kingdom Central Manchester University Hospitals NHS Trust Manchester, United Kingdom, M13 9WL Freeman Hospital Newcastle upon tyne, United Kingdom, NE7 7DN Nottingham University Hospitals NHS Trust Nottingham, United Kingdom, NG7 2UH Derriford Hospital Plymouth, United Kingdom Queen Alexandra Hospital Portsmouth, United Kingdom, PO6 3LY 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.76mL 0.35mL 0.18mL |
8.78mL 1.76mL 0.88mL |
17.56mL 3.51mL 1.76mL |
| 参考文献 |
|---|