| 生物活性 | |||
|---|---|---|---|
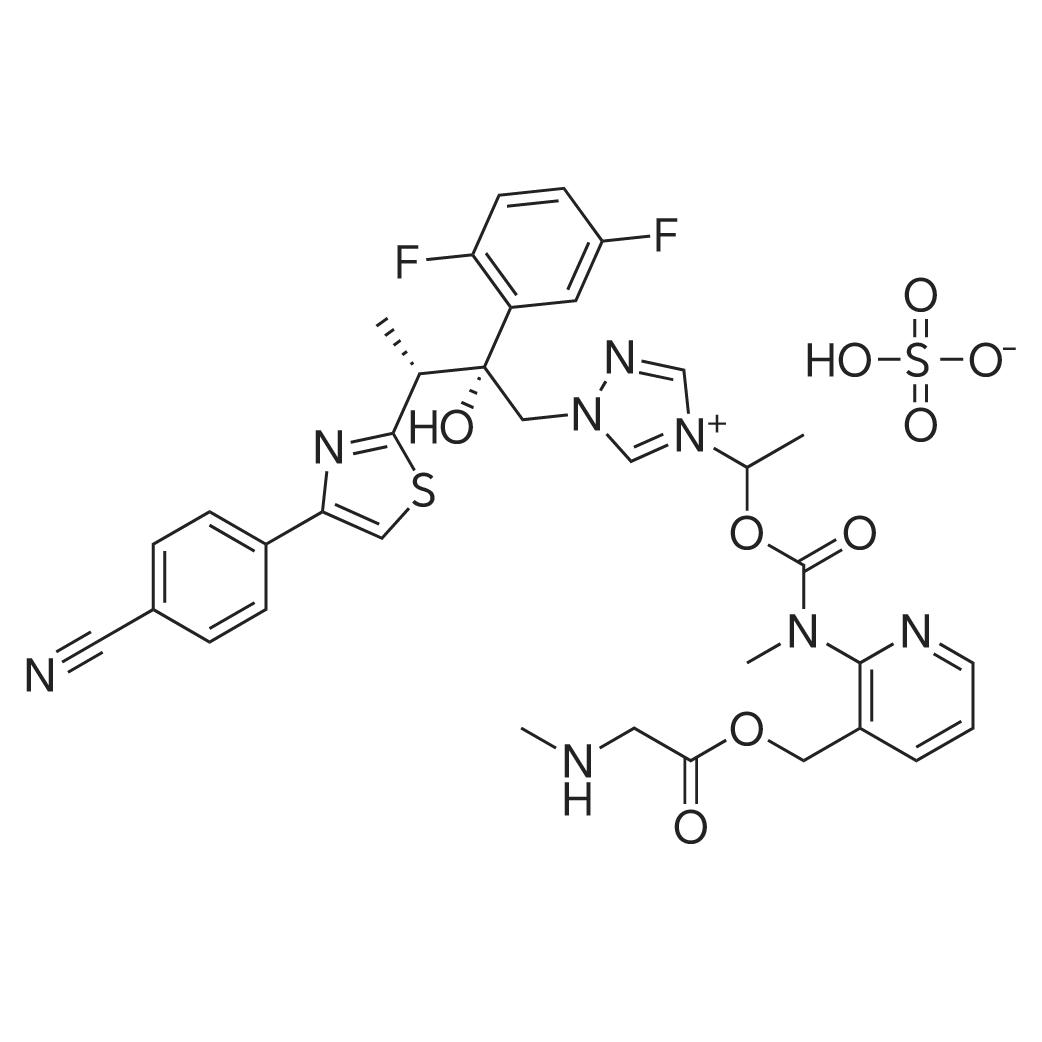
| 描述 | Isavuconazonium sulfate is a prodrug of isavuconazole, a broad-spectrum mould-active triazole antifungal drug. The MIC values obtained for the prodrug were one 2-fold dilution higher than those of isavuconazole for all isolates tested. The essential agreement (±2 log2 dilutions) between prodrug and isavuconazole MIC values was 96.4% across all tested isolates[3]. Isavuconazonium sulfate, is currently approved in the United States and Europe for the treatment of the two of the most common and most challenging invasive fungal infections in clinical practice, invasive aspergillosis and invasive mucormycosis. It is available in both oral and intravenous formulations for once-a-day dosing and has favorable safety profile and drug interaction potential in comparison to voriconazole[4]. And Isavuconazonium sulfate has demonstrable activity against Candida species and other common fungal pathogens[5]. Similar significant reductions in the fungal burden in the brain and cerebrospinal fluid in rabbits treated with isavuconazonium sulfate and fluconazole compared with that in the untreated controls were observed. Isavuconazonium sulfate, like fluconazole, could be beneficial in the setting of consolidation and maintenance therapy, rather than induction monotherapy, in high-burden cryptococcal meningoencephalitis[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.23mL 0.25mL 0.12mL |
6.14mL 1.23mL 0.61mL |
12.27mL 2.45mL 1.23mL |
| 参考文献 |
|---|