| 生物活性 | |||
|---|---|---|---|
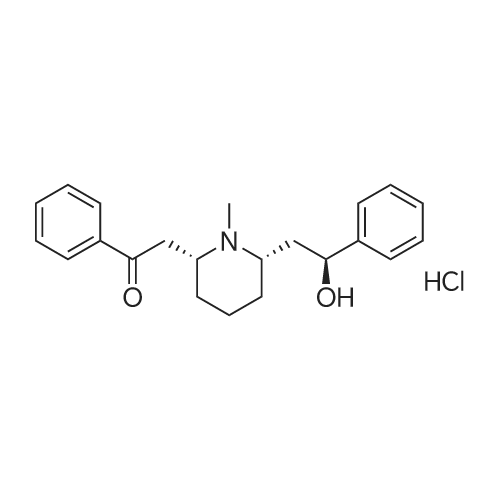
| 描述 | Lobeline hydrochloride, a nicotinic receptor agonist, acting as a potent antagonist at both α3β2 and α4β2 neuronal nicotinic receptor subtypes. The pKa of (-)-lobeline HCl at 25 degrees C is 8.6 (approx), indicating that (-)-lobeline is at least 90% in the protonated form at physiological pH (7.6) [3]. Intravenous injections of lobeline HCl into twenty-six normal young male human volunteers produced sensations of choking, pressure or fumes in the throat and upper chest at a mean threshold dose of 12 micrograms kg-1[4]. Sodium cyanide, lobeline HCl, and doxapram HCl in the doses of 2-400 mug/kg injected into the external carotid artery stimulated respiration significantly. Injections of the drugs into the ascending aorta produced less effects which were abolished after section of the carotid sinus nerves. The drugs produced a significant increase in the carotid sinus nerve activity but failed to do so in the aortic depressor or recurrent laryngeal nerves[5]. Stimulation of carotid body chemoreceptors by i.a. injections of lobeline, doxapram or 0.015 N HCl in saline also briefly reinstates phrenic nerve discharges after inhibition by clonidine. Inhibition is also overcome during electrical stimulation of the carotid sinus nerve[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.67mL 0.53mL 0.27mL |
13.37mL 2.67mL 1.34mL |
26.74mL 5.35mL 2.67mL |
| 参考文献 |
|---|