| 生物活性 | |||
|---|---|---|---|
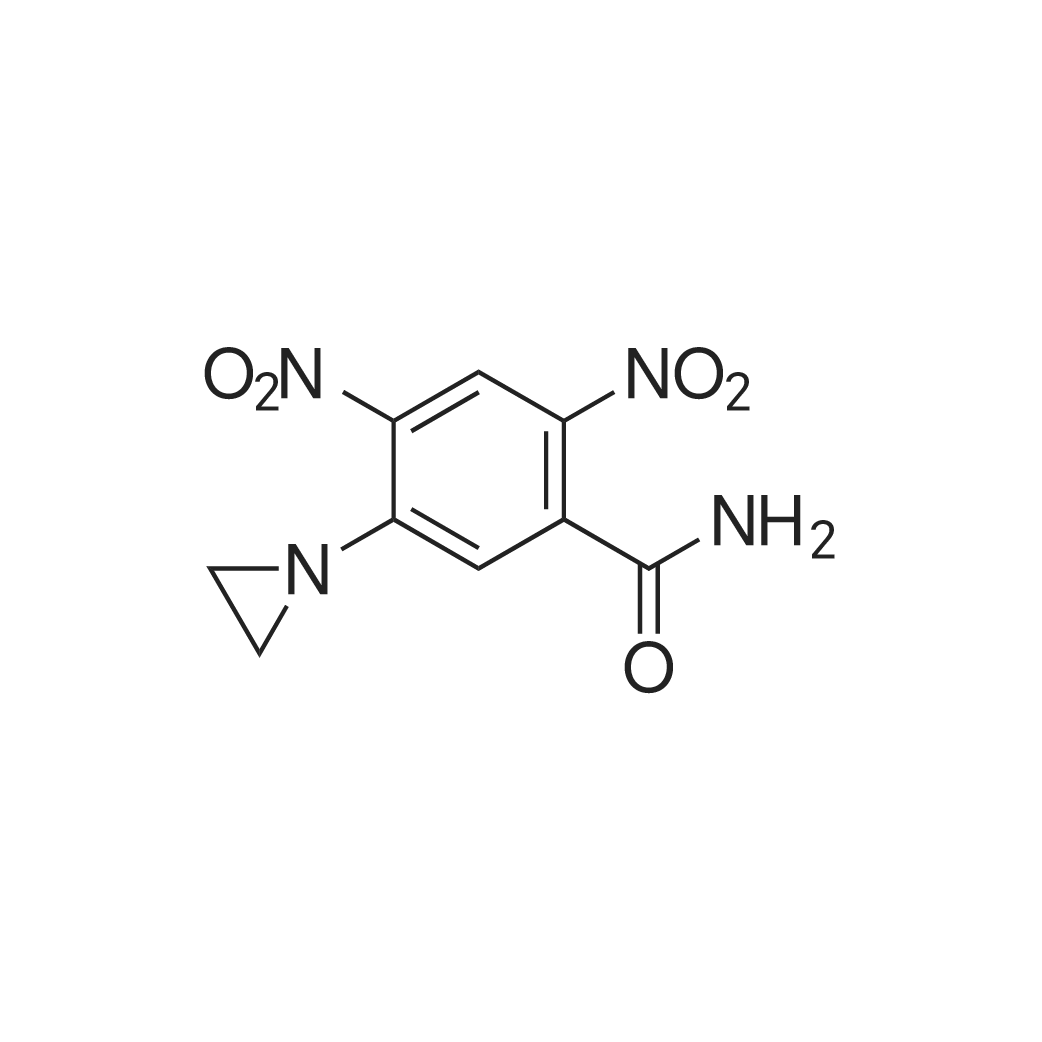
| 描述 | CB 1954 [5-(aziridin-1-yl)-2,4-dinitrobenzamide] is an antitumor prodrug that is activated in certain rat tumors via its 4-hydroxylamine derivative to a potent bifunctional alkylating agent. It is enzymatically activated to generate a difunctional agent, which can form DNA-DNA interstrand crosslinks[2]. The bioactivation of CB 1954 in rat cells involves the aerobic reduction of its 4-nitro group to a 4-hydroxylamine by the enzyme NQO1 (DT-diaphorase)[3].CD-1 nu/nu mice were initially dosed at 200 mol/kg and showed no signs of toxicity, and a higher dose was assessed and the maximum tolerated doses (MTD) was established at 562mol/kg. None of the mice showed overt toxicity at this dose, although weight loss was observed in some animals[4].The formation of CB 1954 metabolites was investigated in the liver, plasma and urine of mice and rats dosed at the respective MTD for 4 h post-dose. In mice dosed at the MTD of 562mol/kg, plasma levels of ALT and AST were raised to 6 and 13 times normal levels, respectively, between 3 and 6 days postdose[5].CB1954 at concentrations of 12.5 and 25 µmol/l increased the sensitization enhancement ratio of HeLa cells to 1.54 and 1.66, respectively. When compared with monotherapy, the combined therapy of NTR/CB1954 and γ‑rays may increase the apoptotic rate and enhance the radiosensitivity of HeLa cells. The combined therapy of γ‑ray radiation and the NTR/CB1954 suicide gene system may be a novel and potent therapeutic method for the treatment of cervical carcinoma[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00746590 | Hepatocellular Carcinoma | Phase 2 | Terminated(Study terminated pr... 展开 >>ematurely by sponsor for business reason. One patient was enrolled.) 收起 << | - | Belgium ... 展开 >> Cliniques Universitaires Saint-Luc Brussels, Belgium, 1200 收起 << |
| NCT00746590 | - | Terminated(Study terminated pr... 展开 >>ematurely by sponsor for business reason. One patient was enrolled.) 收起 << | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.97mL 0.79mL 0.40mL |
19.83mL 3.97mL 1.98mL |
39.65mL 7.93mL 3.97mL |
| 参考文献 |
|---|