| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
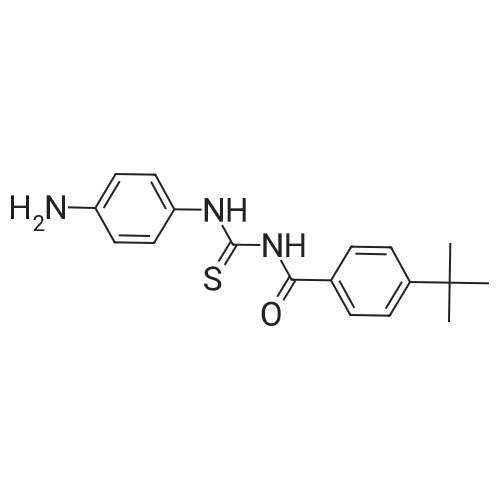
| 描述 | Tenovin-3 is a potent p53 activator. It increase p53 levels, followed increased p21 expression, in MCF-7 cells treated for 6 hr at 10μM, increased K40 Ac-tubulin levels in H1299 cells treated for 16 hr at 10μM and inhibited the deacetylase activities of purified sirt2[4]. The activation of Tenovin-3 on p53 may due to its inhibitory effect on sirtuin[5]. Tenovin-3 exhibited anti-virus activity on vaccinia virus (VV) and La Crosse virus (LACV) with EC50 values of 2.72μM and 2.19μM, respectively[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.05mL 0.61mL 0.31mL |
15.27mL 3.05mL 1.53mL |
30.54mL 6.11mL 3.05mL |
| 参考文献 |
|---|