| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
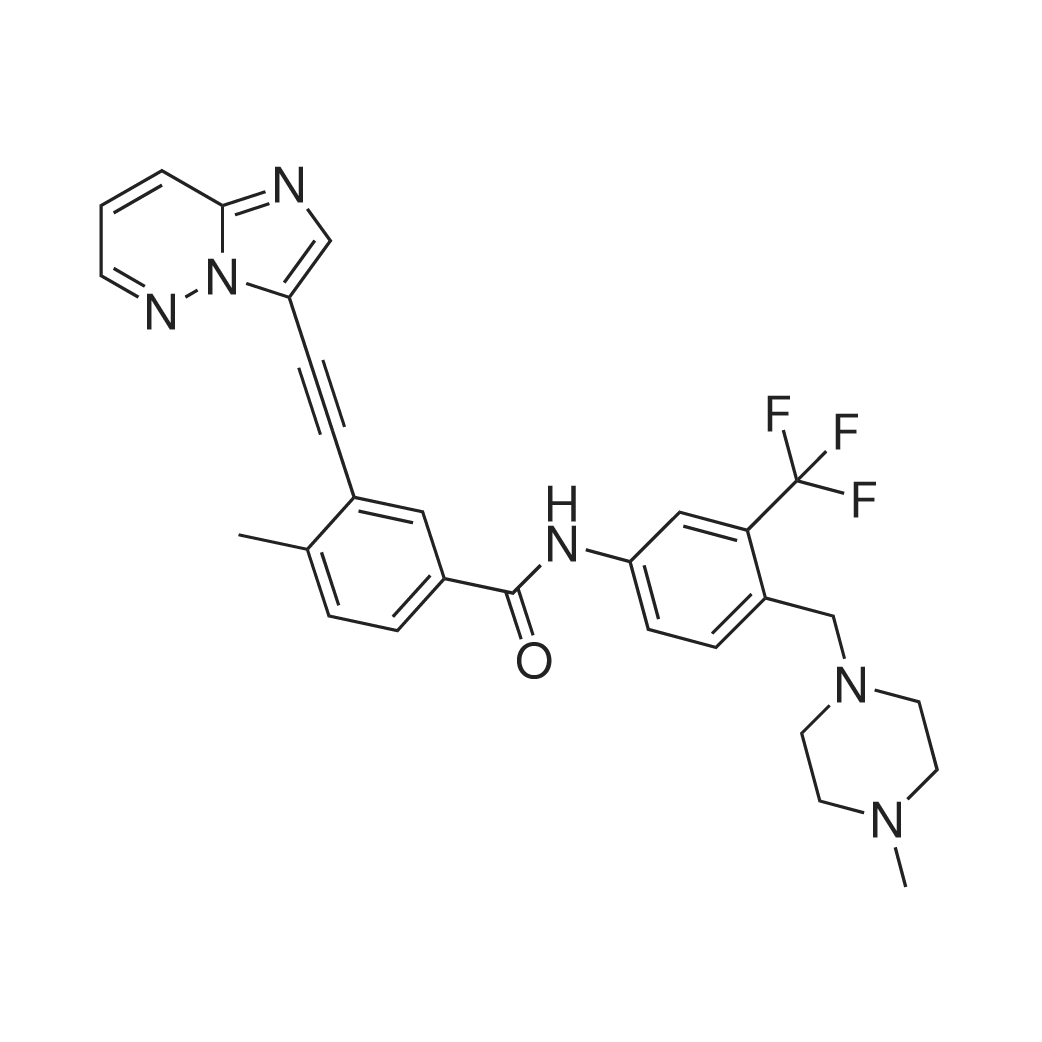
| 描述 | The oncogenic tyrosine kinase Bcr-Abl plays a central role in the pathogenesis of chronic myelogenous leukemia, thus makes it as the therapy drug target. However, it is demonstrated that the mutations of Bcr-Abl kinase, especially the mutation of T315 residue in the gatekeeper region of the ATP-binding site, have been the most common mechanism of drug resistance, such as imatinib, nilotinib and dasatinib. Ponatinib is a multi-target inhibitor with IC50 values of 0.37nM, 1.1nM, 1.5nM, 2.2nM, 5.4nM and 12.5nM for Abl, PDGFRα, VEGFR2, FGFR1, c-Src and c-Kit, respectively. Distinguished with other Bcr-Abl inhibitors like imatinib, nilotinib and dasatinib, Ponatinib was effective against the ABLT315I mutant with significant inhibition on autophosphorylation of Abl and AblT315I mutant at concentration≥100nM, whereas the other compound did not. Consistent with the in vitro study, Ponatinib inhibited the cellular proliferation of Ba/F3 expressing various Bcr-Abl mutations with IC50 ranging in 0.5-36nM, as well as CML leukemia cells K562, KY01 and LAMA cell line with IC50 ranging in 0.3-3.9nM, but anti-proliferative on parental Ba/F3 cells and non CML leukemia cells at micromolar concentration. Treatment with Ponatinib at concentration>100nM for 4h can potently suppress the tyrosine phosphorylation status of BCR-ABL and the direct BCR-ABL substrate CrkL in both Ba/F3 cells expressing native BCR-ABL and Bcr-AblT315I mutant, further confirming the inhibition of Bcr-Abl-mediated signaling in cells expressing Bcr-AblT315I mutant. Oral administration of Ponatinib at dose of 5, 15 and 25mg/kg for 19 days prolonged median survival to 19.5, 26, and 30 days, respectively compared to 16 days for control group in a survival model in which mice were injected with Ba/F3 Bcr-AblT315I cells. Daily oral administration of 50mg/kg Ponatinib caused significant tumor regression with a 96% reduction in mean tumor volume in mice injected subcutaneously with Ba/F3 cells expressing Bcr-AblT315I mutant[1]. | ||
| 作用机制 | Ponatinib can occupy the adenine pocket of Abl, especially making favorable van der Waals interactions with the I315 mutated residue.[1] | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| 22RV1 | Growth Inhibition Assay | IC50=39.8398 μM | SANGER | ||
| 23132-87 | Growth Inhibition Assay | IC50=41.2732 μM | SANGER | ||
| 5637 | Growth Inhibition Assay | IC50=4.90487 μM | SANGER | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02551718 | Acute Leukemia of Ambiguous Li... 展开 >>neage Recurrent Adult Acute Lymphoblastic Leukemia Recurrent Adult Acute Myeloid Leukemia Recurrent Childhood Acute Lymphoblastic Leukemia Recurrent Childhood Acute Myeloid Leukemia Refractory Acute Myeloid Leukemia Refractory Adult Acute Lymphoblastic Leukemia Refractory Childhood Acute Lymphoblastic Leukemia 收起 << | Not Applicable | Recruiting | - | United States, Washington ... 展开 >> Fred Hutch/University of Washington Cancer Consortium Recruiting Seattle, Washington, United States, 98109 Contact: Pamela S. Becker 206-616-1589 pbecker@u.washington.edu Principal Investigator: Pamela S. Becker 收起 << |
| NCT01883219 | Philadelphia Chromosome Positi... 展开 >>ve Acute Lymphocytic Leukemia Stem Cell Transplantation Minimal Residual Disease 收起 << | Phase 2 | Unknown | November 2017 | China, Guangdong ... 展开 >> Department of Hematology,Nanfang Hospital, Southern Medical University Recruiting Guangzhou, Guangdong, China, 510515 Contact: Ren Lin, MD +86-020-61641613 lansinglinren@hotmail.com 收起 << |
| NCT03331211 | - | Recruiting | September 1, 2019 | China, Guangdong ... 展开 >> Department of Hematology,Nanfang Hospital Recruiting Guangzhou, Guangdong, China, 510515 Contact: Xutao Guo 008613802426709 ext 1 gxt827@126.com 收起 << | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.88mL 0.38mL 0.19mL |
9.39mL 1.88mL 0.94mL |
18.78mL 3.76mL 1.88mL |