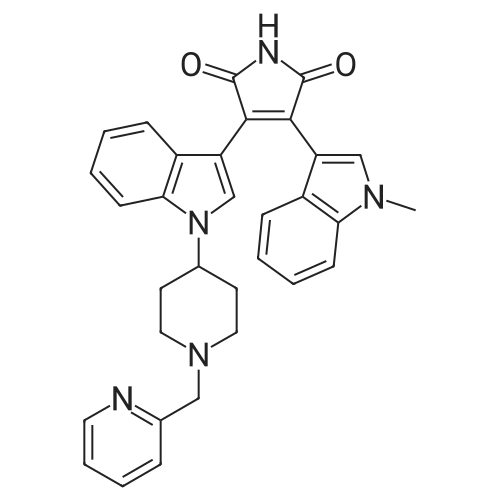
| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
| 描述 | The protein kinase C (PKC) family of serine-threonine protein kinases has been implicated in the processes that control cell proliferation, motility, invasion, and apoptosis. Enzastaurin is an ATP-competitive, selective inhibitor of PKC with IC50 values of 6nM, 39nM, 83nM and 110nM for PKCβ, PKCα, PKCγ, and PKCε, respectively[3]. In vitro, Enzastaurin can inhibit cell proliferation, including H460, LXF289, H1395, A427, A549, and H1299 cell lines with IC50 values ranging in 5 - 12 μM[4]. Enzastaurin suppressed the proliferation of U87MG glioblastoma cells, PC-2 prostate carcinoma cells, and HCT116 colon carcinoma cells with concentrations ranging from 1 – 10 μM. K-562, MOLT-4, HOP-92, and PC-3 were the most sensitive cell lines to enzastaurin. However, Prostate cancer cell line DU-145; Breast cancer cell lines HS-578T, BT-549, and T-47D; Melanoma cell line MALme-3M; Lung cancer cell lines HOP-62, NCIH23, NCI-H322m, and NCI-460. Ovarian cancer cell lines OVCAR-5 and SK-OV-3; The renal cell lines 786-0, A498, ACHN, RXF393, and TK-10 were not affected by Enzastaurin. Enzastaurin induced HT116 and U87MG cell apoptosis in the low micromolar range in 1 – 4 μM. Enzastaurin at 1 μM inhibited the activity of PKCβ, PKCγ, PKCδ, PKCθ, PKCξ, and PKCε by about 90% or more but did not show significant inhibition of PKCμ and PKCι. At 1 μM, enzastaurin also suppressed the activity of p70S6, PKCζ, and PKCη by nearly 50%. Treatment of HCT116 and U87MG cells with 1 μM enzastaurin did not inhibit ERK activity[3]. In vivo, oral administration with enzastaurin at 75 mg/kg twice daily for 21 days significantly suppressed the growth of both HCT116 colon carcinoma and U87MG glioblastoma xenografts in mice. In both cultured HCT116 cells and in xenograft tumor tissues from the same cell lines, enzastaurin treatment suppressed GSK3h-Ser9 phosphorylation, indicating that GSK3h-Ser9 phosphorylation may serve as a reliable marker for enzastaurin activity[3]. The phase I clinical trials for enzastaurin have shown that oral administration at 525 mg/d yields -2 μM mean steady-state plasma exposure of enzastaurin and its analytes. Enzastaurin has successfully advanced to phase II clinical trials for the treatment of refractory glioblastoma and DLBCL[3]. | ||
| 作用机制 | Enzastaurin selectively inhibits PKC activity by interacting competitively at its ATP-binding site[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00052273 | Adult Solid T... 展开 >>umor 收起 << | Phase 1 | Completed | - | United States, California ... 展开 >> Jonsson Comprehensive Cancer Center, UCLA Los Angeles, California, United States, 90095-1781 收起 << |
| NCT00542919 | T-Cell Lymphoma ... 展开 >> B-Cell Lymphoma 收起 << | Phase 2 | Completed | - | United States, California ... 展开 >> For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Anaheim, California, United States, 92801 Australia, Australian Capital Territory For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Garran, Australian Capital Territory, Australia, 2605 Australia, New South Wales For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Gosford, New South Wales, Australia, 2250 Australia, Queensland For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Auchenflower, Queensland, Australia, 4066 Australia, Victoria For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Prahran, Victoria, Australia, 3181 Brazil For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Brasilia, Brazil, 70710-904 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Curitiba, Brazil, 81520-060 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Jau, Brazil, 17210-120 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Porto Alegre, Brazil, 90430-090 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. São Paulo, Brazil, 01323-903 India For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Bangalore, India, 560017 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Chennai, India, 6000 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Kolkata, India, 700053 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Navrangpura, India, 380009 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Secunderabad, India, 800003 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Trivandrum, India, 695011 Mexico For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Monterrey, Mexico, 64460 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Tepic, Mexico, 63000 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Tlalpan, Mexico, 14080 Peru For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Lima, Peru, LIMA13 收起 << |
| NCT03776071 | Glioblastoma | Phase 2 | Not yet recruiting | December 2022 | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.94mL 0.39mL 0.19mL |
9.70mL 1.94mL 0.97mL |
19.39mL 3.88mL 1.94mL |
| 参考文献 |
|---|