| 生物活性 | |||
|---|---|---|---|
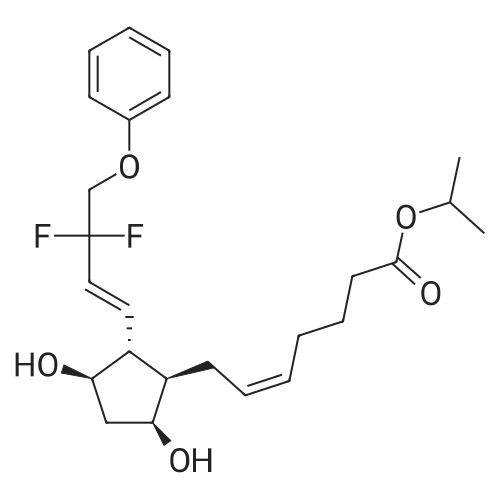
| 描述 | Tafluprost is an anti-glaucoma prostaglandin (PG) analog. Tafluprost showed significant IOP(intraocular pressure)-lowering effects without any safety concerns in patients with various types of glaucoma and OH (ocular hypertension) in daily clinical practice and tafluprost is highly effective in any therapeutic patterns[3]. Tafluprost lowers IOP (intraocular pressure) through the prostanoid FP receptor. A part of ocular hypotensive effect of tafluprost is attributed to FP receptor-mediated prostaglandin production acting through the prostanoid EP3 receptor[4]. Tafluprost not only lowers IOP, but may also enhance retinal blood flow in POAG (Primary open-angle glaucoma) patients with a normal IOP[5]. A significant increase of MOPP (mean ocular perfusion pressure) and a decrease of VD (vessel density) in the ONH (inside disc) in patients with initial glaucoma occurred within a week under the topical tafluprost or its FC (fixed combination)[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01434888 | Healthy Volunteers | Phase 1 | Completed | - | Finland ... 展开 >> Kuopio University Hospital Eye Clinic Kuopio, Finland, 70200 收起 << |
| NCT01654484 | Glaucoma and Ocular Hypertensi... 展开 >>on 收起 << | Phase 1 Phase 2 | Completed | - | United States, California ... 展开 >> Santen Investigational Site Newport Beach, California, United States, 92663 United States, Florida Santen Investigational Site Deerfield Beach, Florida, United States, 33064 Santen Investigational Site Largo, Florida, United States, 33773 United States, Georgia Santen Investigational Site Morrow, Georgia, United States, 30260 Santen Investigational Site Roswell, Georgia, United States, 30076 United States, New York Santen Investigational Site Rochester, New York, United States, 14618 United States, Ohio Santen Investigational Site Cleveland, Ohio, United States, 44115 United States, Texas Santen Investigational Site Austin, Texas, United States, 78731 Santen Investigational Site Fort Worth, Texas, United States, 76102 Santen Investigational Site San Antonio, Texas, United States, 78240 收起 << |
| NCT01927406 | Thyroid Eye Disease ... 展开 >> Ocular Hypertension Glaucoma 收起 << | Phase 4 | Withdrawn(Funding source unava... 展开 >>ilable) 收起 << | December 2016 | United States, California ... 展开 >> Stanford Hospital and Clinics Stanford, California, United States, 94304 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.21mL 0.44mL 0.22mL |
11.05mL 2.21mL 1.10mL |
22.10mL 4.42mL 2.21mL |
| 参考文献 |
|---|