| 生物活性 | |||
|---|---|---|---|
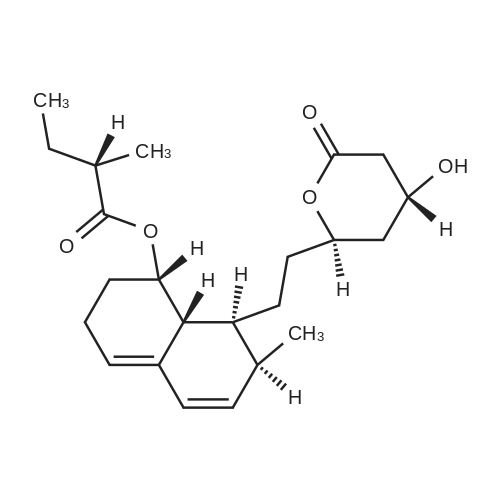
| 描述 | HMG-CoA reductase, the rate-limiting enzyme in the process of cholesterol synthesis in liver cells, catalyzes the production of mevalonate. Inhibiting HMG-CoA reductase inhibits cholesterol synthesis. Mevastatin reversibly inhibits HMG-CoA reductase and competes with HMG-CoA, whose Ki value is about 1 nM[3]. in vitro, at 0.01 pg/mL (26 nM), it reduced cholesterol synthesis by 50% compared with the control group[2]. In addition, mevastatin can completely inhibit L cell growth at 1.3 μM. Mevastatin can also influence cell cycle and change cell morphology in cultured fibroblasts[3]. In vivo, Mevastatin reduced serum cholesterol levels three hours after oral administration at doses of 5 mg/kg and 20 mg/kg. At a dose of 20 mg/kg, it reduces serum cholesterol levels by about 30 percent[2]. Mevastatin reduces cholesterol production in the liver by competitively inhibiting HMG-CoA reductase. Cholesterol levels were reduced only after 28 days of treatment and were not associated with a reduction in infarcts. After 14 days of high-dose treatment, baseline absolute cerebral blood flow was 30% higher[1]. In patients with familial hypercholesterolemia, mevastatin is effective in lowering plasma cholesterol. Treatment at 50 mg/day for 2 months reduced total cholesterol in FH heterozygous patients by 25%[3]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02441400 | - | Recruiting | May 2019 | Argentina ... 展开 >> Fundacion Favaloro Recruiting Buenos Aires, Argentina Contact: Alejandro Nieponice, MD Principal Investigator: Alejandro Nieponice, MD Austria University Hospital Vienna Recruiting Vienna, Austria Contact: Sebastian Schoppmann, MD Principal Investigator: Sebastian Schoppmann, MD Denmark Aarhus University Hospital Recruiting Aarhus, Denmark Contact: Jan B Pedersen, MD Principal Investigator: Jan B Pedersen, MD Germany SANA Klinikum Lichtenberg Recruiting Berlin, Germany Contact: Dirk Federlein Principal Investigator: Dirk Hartmann, MD Evangelisches Krankenhaus Castrop-Rauxel Recruiting Castrop Rauxel, Germany Contact: Pravin Thattamparambil, MD Principal Investigator: Pravin Thattamparambil, MD St. Marienstift Krankenhaus Friesoythe Recruiting Friesoythe, Germany Contact: Ralf Weise, MD Principal Investigator: Ralf Weise, MD Hospital zum Heiligen Geist Fritzlar Recruiting Fritzlar, Germany Contact: Carsten Bismarck Principal Investigator: Carsten Bismarck, MD Klinikum Garmisch-Partenkirchen Recruiting Garmisch-Partenkirchen, Germany Contact: Holger Vogelsang Principal Investigator: Holger Vogelsang, MD Asklepios Klinik Altona Recruiting Hamburg, Germany Contact: Gero Puhl Principal Investigator: Gero Puhl KRH Klinikum Siloah Hannover Oststadt Recruiting Hannover, Germany Contact: Ahmed Madisch Principal Investigator: Ahmed Madisch, MD Evangelisches Krankenhaus Herne Recruiting Herne, Germany Contact: Matthias Kemen, MD Principal Investigator: Matthias Kemen, MD Klinikum Konstanz Recruiting Konstanz, Germany Contact: Ulrike Petzsche Principal Investigator: Marcus Schuchmann, MD Heilig Geist-Krankenhaus Köln Recruiting Köln, Germany Contact: Ernst Eypasch, MD Principal Investigator: Ernst Eypasch, MD Universitätsklinikum Magdeburg Recruiting Magdeburg, Germany Contact: Frank Benedix Principal Investigator: Ulrike von Arnim Universitätsmedizin Mannheim Recruiting Mannheim, Germany Contact: Mirko Otto Principal Investigator: Kai Nowak, MD Klinikum Memmingen Recruiting Memmingen, Germany Contact: Heinz Schlosser Principal Investigator: Carsten Gutt, MD Friedrich-Ebert-Krankenhaus Neumünster Recruiting Neumünster, Germany Contact: Andrea Pace Principal Investigator: Andrea Pace, MD Asklepios Schwalm-Eder Kliniken GmbH Recruiting Schwalmstadt, Germany Contact: Norbert Hesselbarth Principal Investigator: Felix Meuschke, MD Jung Stilling - Siegen Recruiting Siegen, Germany Contact: Daniela-Patricia Borkenstein Principal Investigator: Joachim Labenz, MD St. Marien-Krankenhaus Siegen Recruiting Siegen, Germany Contact: Dietmar Stephan, MD Principal Investigator: Dietmar Stephan, MD Ev. Krankenhaus Wesel GmbH Recruiting Wesel, Germany Contact: Vivianda Menke Principal Investigator: Vivianda Menke, MD Mexico Hospital San José Recruiting Monterrey, Mexico Contact: David Aguirre Mar, MD Principal Investigator: David Aguirre Mar, MD Netherlands Maastricht University Medical Center Recruiting Maastricht, Netherlands Contact: Nicole Bouvy, MD Principal Investigator: Nicole Bouvy, MD United Kingdom Spire Leicester Hospital Recruiting Leicester, United Kingdom Contact: Chris Sutton Principal Investigator: Chris Sutton, MD University Hospital Southampton Recruiting Southampton, United Kingdom Contact: Abrie Botha Principal Investigator: Abrie Botha, MD 收起 << | |
| NCT01382511 | Pachyonychia Congenita | Not Applicable | Unknown | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.56mL 0.51mL 0.26mL |
12.80mL 2.56mL 1.28mL |
25.61mL 5.12mL 2.56mL |
| 参考文献 |
|---|
|
[3]The discovery and development of HMG-CoA reductase inhibitors |