| 生物活性 | |||
|---|---|---|---|
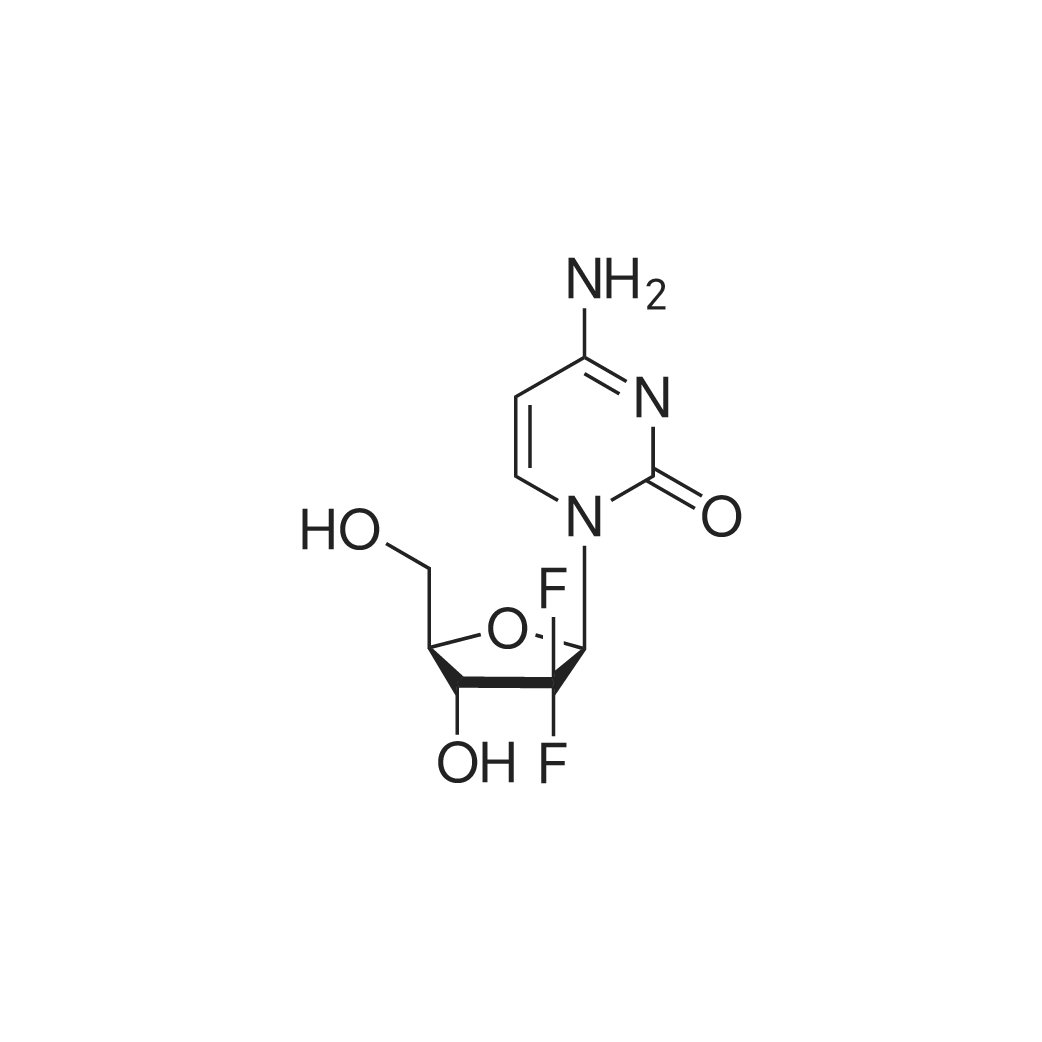
| 描述 | Gemcitabine (dFdCTP, LY-188011, NSC 613327, (+)-2'-Deoxy-2',2'-difluorocytidine) is a deoxycytidine analogue that has a spectrum of activity against solid tumors[1]. Gemcitabine can inhibit DNA synthesis through a combination of two actions. The first, gemcitabine can inhibit ribonucleotide reductase (RR) and then cause the decrease of the deoxynucleotide pool sizes for DNA repair and synthesis[2]. The second, gemcitabine inhibit DNA synthesis through competition with dCTP and incorporation into DNA[3]. Gemcitabine can induce S-phase arrest and cell growth inhibition[4]. | ||
| 作用机制 | Gemcitabine, as nucleoside analogue, can inhibit DNA synthesis through inhibition of ribonucleotide reductase and incorporation into DNA[5]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活动说明 | 数据源 |
| 23132-87 | Growth Inhibition Assay | IC50=43.05 nM | SANGER | ||
| 647-V | Growth Inhibition Assay | IC50=0.248 nM | SANGER | ||
| 697 | Growth Inhibition Assay | IC50=6.25 nM | SANGER | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02240238 | Solid Tumors | Phase 1 Phase 2 | Recruiting | November 30, 2019 | United States, California ... 展开 >> California Cancer Associates for Research and Excellence Recruiting Encinitas, California, United States, 92024 UC San Diego Moores Cancer Center Recruiting La Jolla, California, United States, 92037 Pacific Hematology Oncology Associates Recruiting San Francisco, California, United States, 94115 United States, Illinois Northwestern University Feinberg School of Medicine Recruiting Chicago, Illinois, United States, 60611 United States, Massachusetts Tufts Medical Center Recruiting Boston, Massachusetts, United States, 02111 United States, North Carolina University of North Carolina at Chapel Hill Recruiting Chapel Hill, North Carolina, United States, 27599 United States, Ohio University Hospitals Case Medical Center Recruiting Cleveland, Ohio, United States, 44121 United States, Oklahoma University of Oklahoma Health Sciences Center Recruiting Oklahoma City, Oklahoma, United States, 73104 United States, Texas University of Texas Southwestern Medical Center Recruiting Dallas, Texas, United States, 75390 MD Anderson Cancer Center Recruiting Houston, Texas, United States, 77030 Bulgaria Multiprofile Hospital for Active Treatment Serdika EOOD Recruiting Sofia, Sofia-Grad, Bulgaria, 1632 Complex Oncology Center - Shumen EOOD Recruiting Shumen, Bulgaria, 9700 Italy Istituto Scientifico Romagnolo Per Lo Studio E La Cura Dei Tumori IRST Recruiting Meldola, Italy, 47014 ASST Grande Ospedale Metropolitano Niguarda - Presidio Ospedaliero Ospedale Niguarda Ca' Granda Recruiting Milano, Italy, 20162 Poland Wojewodzki Szpital Specjalistyczny im. Ludwika Rydygiera w Krakowie Recruiting Krakow, Poland, 31826 Med-Polonia Sp. z o.o. Recruiting Poznan, Poland, 60693 Romania Fundeni Clinical Institute Recruiting Bucharest, Romania, 22328 Coltea Clinical Hospital Recruiting Bucharest, Romania, 30171 Prof Dr I Chiricuta Institute of Oncology Recruiting Cluj-Napoca, Romania, 400015 Oncology Center Sfantul Nectarie Recruiting Craiova, Romania, 200347 Euroclinic Oncology Center SRL Recruiting Iasi, Romania, 700106 Institutul Regional de Oncologie Iasi Recruiting Iasi, Romania, 700483 收起 << |
| NCT00610740 | - | Completed | - | - | |
| NCT00610740 | Cervical Cancer ... 展开 >> Endometrial Cancer Ovarian Cancer 收起 << | Phase 2 | Completed | - | United States, Minnesota ... 展开 >> University of Minnesota Cancer Center Minneapolis, Minnesota, United States, 55455 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.80mL 0.76mL 0.38mL |
19.00mL 3.80mL 1.90mL |
37.99mL 7.60mL 3.80mL |
| 参考文献 |
|---|