| 生物活性 | |||
|---|---|---|---|
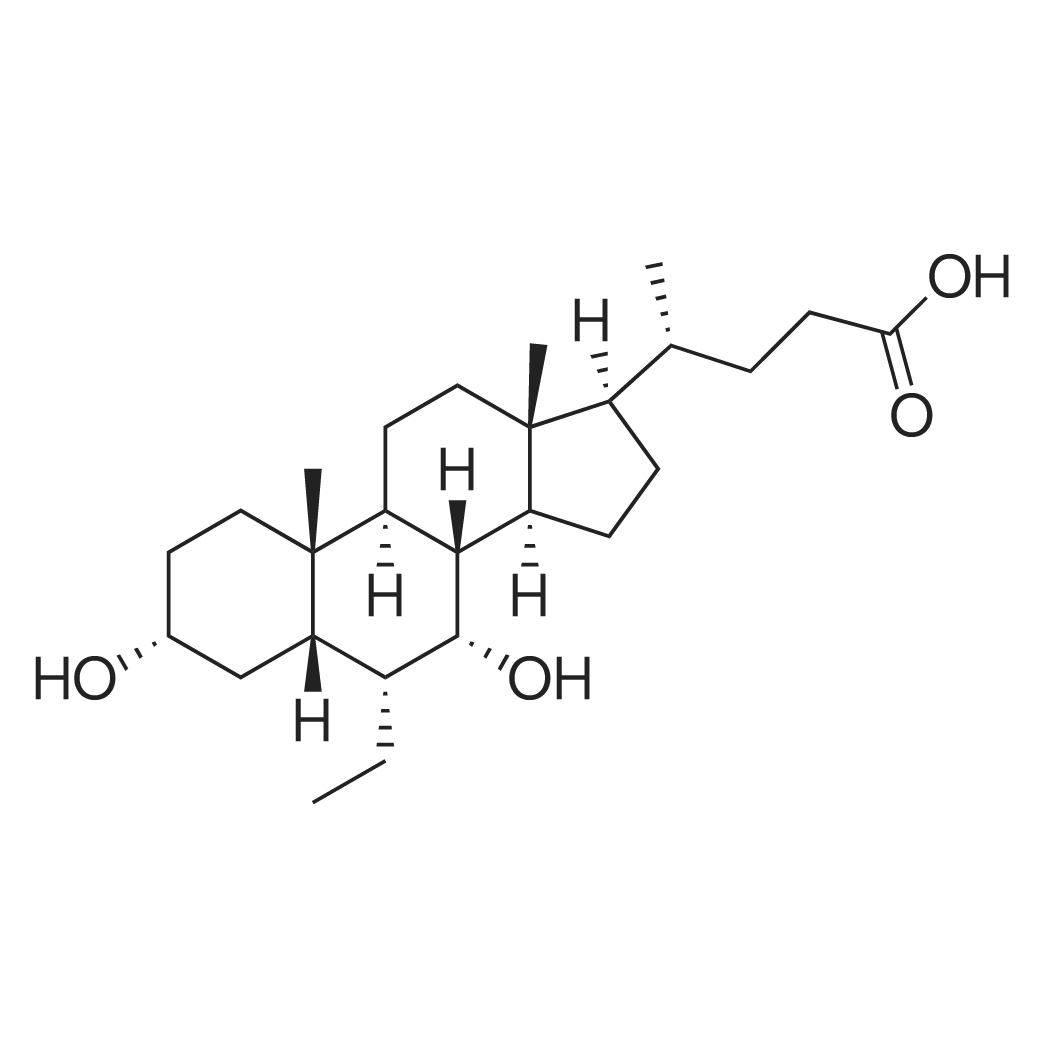
| 描述 | The farnesoid X receptor (FXR) is an orphan member of the nuclear receptor superfamily that can be activated by small, lipophilic hormones. Obeticholic acid is a potent and selective FXR agonist with an EC50 value of 99 nM. It also showed potent FXR agonist activity in HuH7 cells with an EC50 value of 85 nM[3]. The treatment of rat hepatocytes with 1 μM obeticholic acid increased mRNA expression of small heterodimer partner and bile salt export pump. The exposure to obeticholic acid also led to 70 - 80% reduction of cholesterol 7α-hydroxylase, oxysterol 12β-hydroxylase, and Na+/taurocholate cotransporting peptide in rat hepatocytes[4]. The dosing of obeticholic acid at a rate of 3 µmol/kg/min completely reversed the impairment of bile flow and protected rats against lithocholic-induced liver cell injury[3]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| CHO cells | Function assay | 5 h | Agonist activity at human TGR5 expressed in CHO cells after 5 hrs by CRE-driven luciferase reporter gene assay, EC50=0.755 μM | 17685603 | |
| COS1 cells | Function assay | Agonist activity at FXR expressed in COS1 cells by cell-based bioluminescence assay, EC50=99 nM | 20014870 | ||
| HeLa cells | Function assay | 24 h | Agonist activity at human full length FXR expressed in HeLa cells cotransfected with pSG5-human RXR after 24 hrs by Dual-Glo luciferase reporter gene assay, EC50=0.16 μM | 25934227 | |
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02654236 | Alcohol Consumption | Not Applicable | Recruiting | July 2019 | United States, Indiana ... 展开 >> Indiana University Recruiting Indianapolis, Indiana, United States, 46202 Contact: Jennifer Lehman, RN 317-278-1872 jgeck@iu.edu Contact: Megan Comerford, BS, MPH 3172789226 mcomerfo@iu.edu Sub-Investigator: Naga Chalasani, MD Principal Investigator: Suthat Liangpunsakul, MD Sub-Investigator: David Crabb, MD 收起 << |
| NCT00501592 | Diabetes Mellitus, Type II ... 展开 >> Fatty Liver 收起 << | Phase 2 | Completed | - | United States, California ... 展开 >> Profil Institute for Clinical Research, Inc. Chula Vista, California, United States, 91911 UC San Diego VAMC San Diego, California, United States, 92161 United States, Texas Diabetes & Glandular Disease Research Associates, Inc. San Antonio, Texas, United States, 78229 United States, Virginia Virginia Commonwelath University Richmond, Virginia, United States, 23298 收起 << |
| NCT00570765 | Liver Cirrhosis, Biliary | Phase 2 | Completed | - | United States, Michigan ... 展开 >> Henry Ford Novi, Michigan, United States, 39450 United States, Texas Baylor College of Medicine Houston, Texas, United States, 77030 United States, Virginia McGuire DVAMC Richmond, Virginia, United States, 23219 United States, Washington Virginia Mason Medical Center Seattle, Washington, United States, 98101 Austria Karls-Franzens University Graz, Austria, A8036 Canada, Alberta University of Alberta Edmonton, Alberta, Canada, T6G 2C8 Canada, Ontario University of Toronto Toronto, Ontario, Canada, M5T 2S8 Canada, Quebec Centre de Recherche du CHUM / University of Montreal Montreal, Quebec, Canada, H2X 1P1 France Hopital de l'Hotel Dieu Lyon, France, 69288 Hopital Saint-Antoine Paris, France, 75012 Germany Johann Wolfgang Goethe University Frankfurt, Germany, 60590 University Medical Centre Hamburg-Eppendorf Hamburg, Germany, D20246 Medical School of Hannover Hannover, Germany, 30623 University of Munich Munich, Germany, D81377 Spain Hospital Clinic i Provincial Barcelona, Spain, 08036 United Kingdom Queen Elizabeth Medical Center Edgbaston, Birmingham, United Kingdom, B15 2TH Royal Free Hospital Hampstead, London, United Kingdom, NW3 2QG John Radcliffe Hospital Headington, Oxford, United Kingdom, OX3 9DU Royal Infirmary Edinburgh, United Kingdom, EH16 4SA University Upon Tyne/Newcastle Newcastle Upon Tyne, United Kingdom, NE2 4HH 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.38mL 0.48mL 0.24mL |
11.89mL 2.38mL 1.19mL |
23.77mL 4.75mL 2.38mL |
| 参考文献 |
|---|