| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
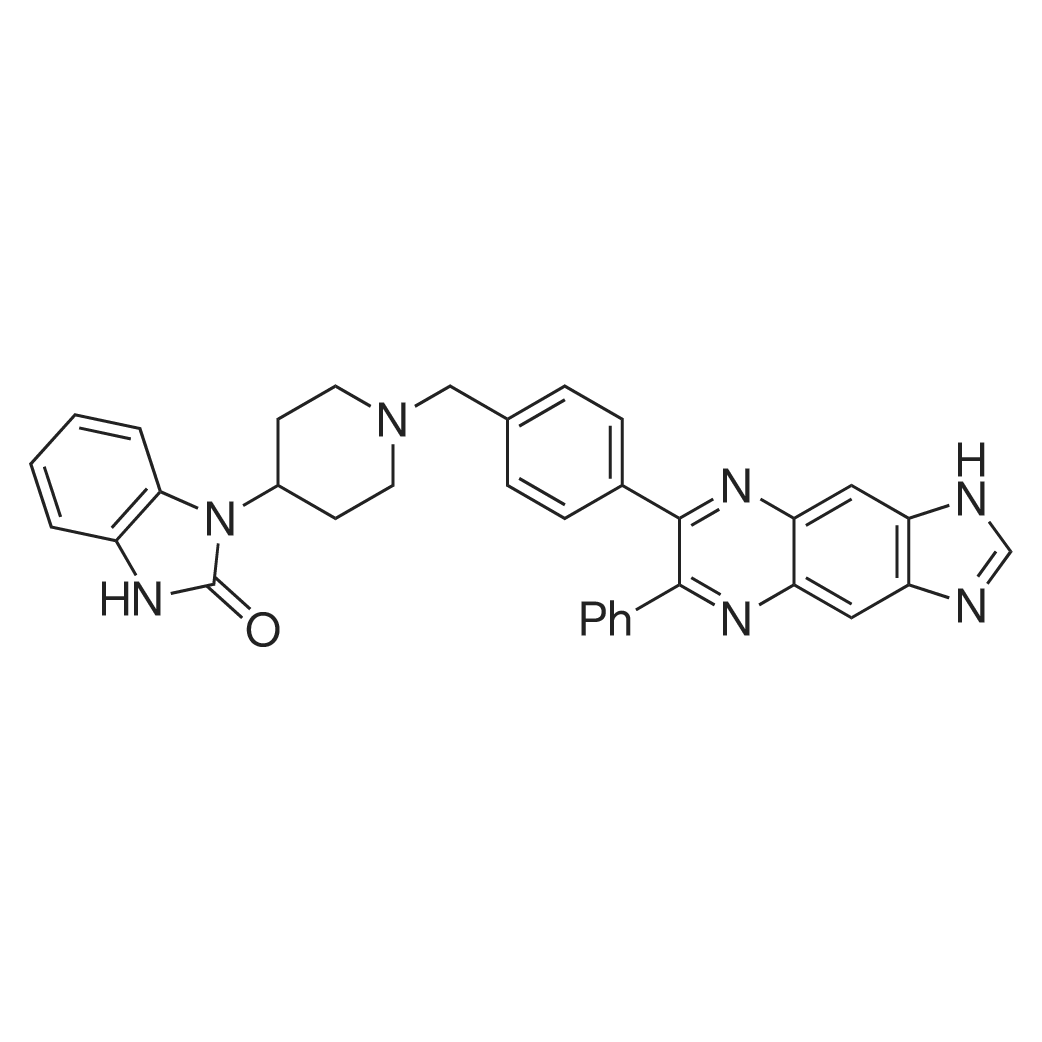
| 描述 | Akt/PKB (protein kinase B) is a serine/threonine kinase which has a key role in the regulation of survival and proliferation. There are three isoforms of human Akt (Akt1, Akt2 and Akt3) and they all have an N-terminal PH (pleckstrin homology) domain and a kinase domain separated by a 39-amino-acid hinge region[3]. AKT inhibitor VIII(Akti-1/2), a cell-permeable quinoxaline compound, is an non-ATP-competitive inhibitor with IC50s of 58 nM, 210 nM, and 2119 nM towards Akt1, Akt2, and Akt3, respectively[4]. This inhibitor prevents phosphorylation of PKB isoforms(Akt1, Akt2 and Akt3), possibly by interfering with the PH domain and/or hinge region of PKB. In vivo study, it was showed that Akti-1/2 can completely inhibit thrombin-mediated phosphorylation of PKB and its downstream substrate GSK3β at a concentration of 5 μM[5]. Akti-1/2 treatment induced an increase in NOXA protein levels and a down-regulation of the levels of MCL-1, a critical survival protein in chronic lymphocytic leukemia (CLL) cells, which indicated that Akti-1/2 might be a new therapeutic option for the treatment of CLL[6]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| 184B5 cells | 20 μM | Cytotoxicity assay | Cytotoxicity against human 184B5 cells at 20 uM chloroquine by SRB assay, GI50=25.76 μM | 18691894 | |
| 22RV1 cell | Growth inhibition assay | Inhibition of human 22RV1 cell growth in a cell viability assay, IC50=6.61424 μM | SANGER | ||
| 23132-87 cell | Growth inhibition assay | Inhibition of human 23132-87 cell growth in a cell viability assay, IC50=0.75938 μM | SANGER | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01257594 | Brain Cancer | Not Applicable | Unknown | December 2016 | United States, New Jersey ... 展开 >> Memorial Sloan-Kettering at Basking Ridge Basking Ridge, New Jersey, United States, 07920 United States, New York Memorial Sloan-Kettering Cancer Center at Commack Commack, New York, United States, 11725 Columbia University Medical Center New York, New York, United States, 10032 Memorial Sloan Kettering Cancer Center New York, New York, United States, 10065 收起 << |
| NCT00897663 | - | Completed | - | United States, Arizona ... 展开 >> Mayo Clinic Scottsdale Scottsdale, Arizona, United States, 85259-5499 United States, Florida Mayo Clinic - Jacksonville Jacksonville, Florida, United States, 32224 United States, Iowa Siouxland Hematology-Oncology Associates, LLP Sioux City, Iowa, United States, 51101 Mercy Medical Center - Sioux City Sioux City, Iowa, United States, 51102 St. Luke's Regional Medical Center Sioux City, Iowa, United States, 51104 United States, Minnesota Mayo Clinic Cancer Center Rochester, Minnesota, United States, 55905 United States, North Dakota Medcenter One Hospital Cancer Care Center Bismarck, North Dakota, United States, 58501 Mid Dakota Clinic, PC Bismarck, North Dakota, United States, 58501 St. Alexius Medical Center Cancer Center Bismarck, North Dakota, United States, 58502 United States, South Dakota Rapid City Regional Hospital Rapid City, South Dakota, United States, 57701 收起 << | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.81mL 0.36mL 0.18mL |
9.06mL 1.81mL 0.91mL |
18.13mL 3.63mL 1.81mL |
| 参考文献 |
|---|