| 生物活性 | |||
|---|---|---|---|
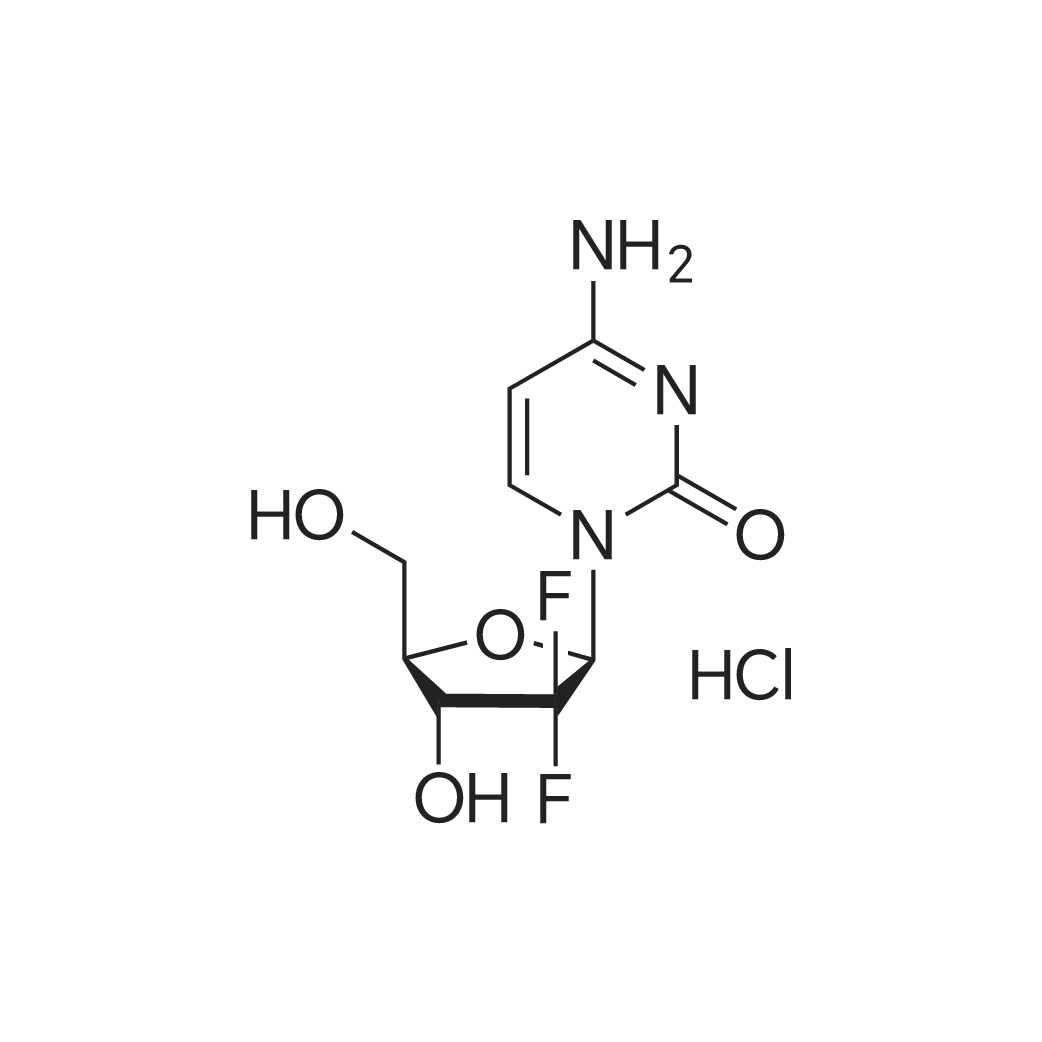
| 描述 | Gemcitabine hydrochloride is the hydrochloride form of Gemcitabine. Gemcitabine (dFdCTP, LY-188011, NSC 613327, (+)-2'-Deoxy-2',2'-difluorocytidine) is a deoxycytidine analogue that has a spectrum of activity against solid tumors[1]. Gemcitabine can inhibit DNA synthesis through a combination of two actions. The first, gemcitabine can inhibit ribonucleotide reductase (RR) and then cause the decrease of the deoxynucleotide pool sizes for DNA repair and synthesis[2][3]. The second, gemcitabine inhibit DNA synthesis through competition with dCTP and incorporation into DNA[4][4]. Gemcitabine can induce S-phase arrest and cell growth inhibition[6]. | ||
| 作用机制 | Gemcitabine, as nucleoside analogue, can inhibit DNA synthesis through inhibition of ribonucleotide reductase and incorporation into DNA.[2][3] | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| CCRF-CEM | Growth Inhibition Assay | IC50=2.9 ± 1.8 nM | 22425885 | ||
| CCRF-CEM | Growth Inhibition Assay | IC50=2.9 ± 1.8 nM | 22425885 | ||
| CCRF-CEM | Growth Inhibition Assay | 72 h | IC50=2.0 ± 0.6 nM | 21851843 | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.34mL 0.67mL 0.33mL |
16.69mL 3.34mL 1.67mL |
33.37mL 6.67mL 3.34mL |
| 参考文献 |
|---|