| 生物活性 | |||
|---|---|---|---|
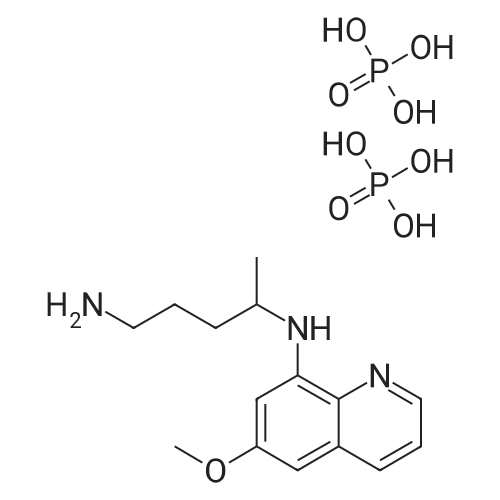
| 描述 | Primaquine (Diphosphate) is the only generally available anti-malarial that prevents relapse in vivax and ovale malaria, and the only potent gametocytocide in falciparum malaria. The currently recommended WHO single low dose (0.25 mg base/kg) to block falciparum malaria transmission confers a very low risk of haemolytic toxicity[3]. Primaquine (PQ) prevents relapses of vivax malaria but may induce severe haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficient patients. The risks of using primaquine in infancy must be weighed against the risks of recurrent vivax malaria in early life[4]. Primaquine is also effective against all exoerythrocytic forms of the parasite and is used in conjunction with other anti-malarials for the treatment of vivax and ovale malaria. However, primaquine is often associated with serious adverse effects, in consequence of its toxic metabolites[5]. A regimen of 0.5 mg/kg primaquine daily for 14 days, on the basis of superior efficacy and good tolerability and safety in nonpregnant persons without glucose-6-phosphate dehydrogenase deficiency[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01659281 | Plasmodium Falciparum Malaria | Not Applicable | Completed | - | Thailand ... 展开 >> Vector Borne Diseases Control Units (VBDC, malaria clinics) Borai, Khaosaming and Muang districts, Trat, Thailand, 23000 收起 << |
| NCT02324738 | Healthy | Phase 4 | Completed | - | Thailand ... 展开 >> Faculty of Tropical Medicine Bangkok, Thailand, 10400 收起 << |
| NCT02192944 | Healthy | Phase 4 | Completed | - | Thailand ... 展开 >> Faculty of Tropical Medicine, Mahidol University Bangkok, Thailand, 10400 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.20mL 0.44mL 0.22mL |
10.98mL 2.20mL 1.10mL |
21.96mL 4.39mL 2.20mL |
| 参考文献 |
|---|
|
[6]Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39(9):1336‐1345 |