| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
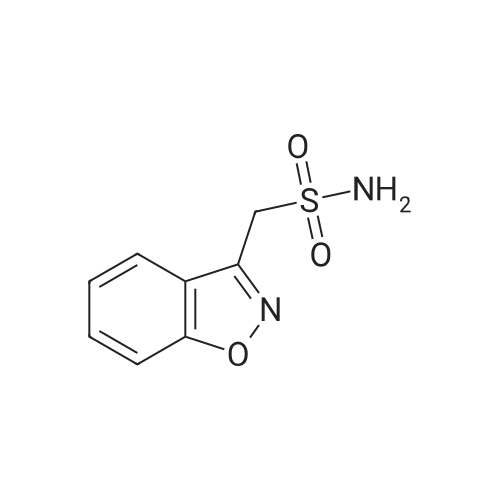
| 描述 | Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug. It has shown activity in various animal models of epilepsy, and although a detailed mode of action awaits clarification it appears to block the propagation/spread of seizure discharges and to suppress the epileptogenic focus. Animal studies suggest that zonisamide possesses a more favourable therapeutic index than most other antiepileptic drugs[3]. Zonisamide may have some utility, especially as an adjunctive therapy, for the management of acute phases and weight gain in bipolar disorder and for prevention of alcohol misuse[4]. Moreover, zonisamide combination therapy was beneficial in treating motor symptoms in PD patients receiving antiparkinson drugs and was well tolerated in Japanese patients[5]. Administration of zonisamide (20 mg/kg, i.p. every 4 h × 3) following a single injection of MPTP (12.5 mg/kg, s.c.) reduced microglial Nav 1.6 and microglial activation in the striatum, as indicated by Iba-1 staining and mRNA expression of F4/80. Zonisamide may reduce neuroinflammation through the down-regulation of microglial Nav 1.6[6]. Zonisamide (adjunctive to levodopa) improved parkinsonism accompanying DLB (dementia with Lewy bodies) without worsening cognitive function or psychiatric symptoms[7]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
4.71mL 0.94mL 0.47mL |
23.56mL 4.71mL 2.36mL |
47.12mL 9.42mL 4.71mL |
| 参考文献 |
|---|