| 生物活性 | |||
|---|---|---|---|
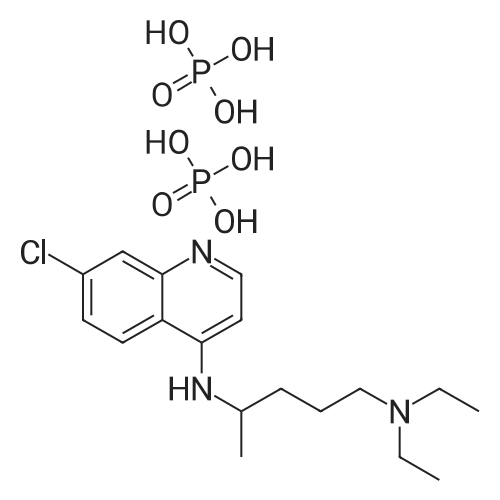
| 描述 | Chloroquine diphosphate (CQ), an autophagy inhibitor that may enhance the cytocidal effect of gefitinib against cSCC, was used in the present study. Suppression of autophagy by CQ, which was demonstrated by an alteration in microtubule associated protein 1 light chain 3-B in CQ pre-treated A431 cells, significantly enhanced cell apoptosis, which suggested that gefitinib-induced autophagy is cytoprotective. CQ was demonstrated to exhibit a synergistic apoptotic effect when used in combination with gefitinib during cSCC(cutaneous squamous cell carcinoma) therapy[2]. In vitro studies indicate that the antiviral effect of chloroquine diphosphate (CQ) requires a high concentration of the drug[3]. Chloroquine diphosphate (25mg/kg and 50mg/kg, respectively) significantly inhibited the growth of the implanted 4T1 tumor cells and induced apoptosis in the tumor microenvironment. Moreover, the metastasis of tumor cells to the lungs was inhibited significantly and the survival of the mice enhanced[4]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| human HeLa cells | Cytotoxic assay | Cytotoxicity against human HeLa cells assessed as growth inhibition by MTT assay, IC50=30 μM | 24354322 | ||
| human HepG2 cells | Cytotoxic assay | 48 h | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50=37.68 μM | 23815186 | |
| human K562 cells | Cytotoxic assay | Cytotoxicity against human K562 cells by MTT assay, IC50=31.83 nM | 18538567 | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02563496 | Malaria, Vivax | Phase 2 | Recruiting | November 30, 2019 | Colombia ... 展开 >> GSK Investigational Site Recruiting Monteria, Colombia Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Center +44 (0) 20 8990 4466 GSKClinicalSupportHD@gsk.com Thailand GSK Investigational Site Not yet recruiting Tak, Thailand, 63110 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Center +44 (0) 20 8990 4466 GSKClinicalSupportHD@gsk.com Vietnam GSK Investigational Site Recruiting Hanoi, Vietnam, 100000 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Center +44 (0) 20 8990 4466 GSKClinicalSupportHD@gsk.com GSK Investigational Site Recruiting Ho Chi Minh City, Vietnam, 700000 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Center +44 (0) 20 8990 4466 GSKClinicalSupportHD@gsk.com GSK Investigational Site Recruiting Ho Chi Minh, Vietnam Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Center +44 (0) 20 8990 4466 GSKClinicalSupportHD@gsk.com 收起 << |
| NCT01136850 | Malaria in Pregnancy ... 展开 >> Sexually Transmitted Infections Anaemia 收起 << | Phase 3 | Completed | - | Papua New Guinea ... 展开 >> Papua New Guinea Institute of Medical Research Madang, Madang Province, Papua New Guinea 收起 << |
| NCT00157859 | Falciparum Malaria ... 展开 >> Vivax Malaria 收起 << | Not Applicable | Completed | - | Indonesia ... 展开 >> SP9 & SP12 Public Health- Malaria control clinics Timika, Papua, Indonesia 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.94mL 0.39mL 0.19mL |
9.69mL 1.94mL 0.97mL |
19.39mL 3.88mL 1.94mL |
| 参考文献 |
|---|