| 生物活性 | |||
|---|---|---|---|
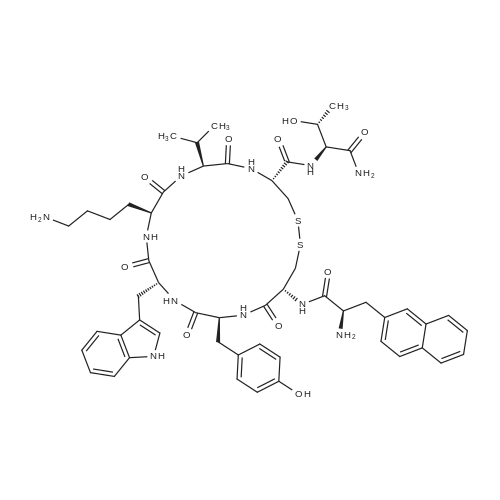
| 描述 | Lanreotide (LAN), a synthetic cyclic octapeptide, is the analogue of somatostatin that can inhibit somatostatin receptor 2 (SSTR-2). Because its half-life is longer than somatostatin, lanreotide can be used clinically to treat neuroendocrine tumors that secrete excessive amounts of growth hormone (acromegaly) or other active hormones or neuropeptides[3]. Lanreotide treatment promoted a Th1 cytotoxic immune-phenotype in patients with NETs (neuroendocrine tumors) originated by intestinal sites[4]. Lanreotide was associated with significantly prolonged progression-free survival among patients with metastatic enteropancreatic neuroendocrine tumors of grade 1 or 2[5]. Lanreotide treatment for 3 months before trans-sphenoidal surgery effectively reduced tumour size, and improved surgical cure rate, in newly diagnosed patients with acromegaly resulting from invasive pituitary macroadenoma[6]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02668172 | Acromegaly | Phase 4 | Unknown | June 2017 | Netherlands ... 展开 >> Erasmus Medical Center Rotterdam, South Holland, Netherlands, 3000CA 收起 << |
| NCT01677910 | Carcinoid Syndrome | Phase 3 | Completed | - | - |
| NCT00642720 | Quality of Life ... 展开 >> Acromegaly 收起 << | Phase 4 | Completed | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
0.91mL 0.18mL 0.09mL |
4.56mL 0.91mL 0.46mL |
9.12mL 1.82mL 0.91mL |
| 参考文献 |
|---|