| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
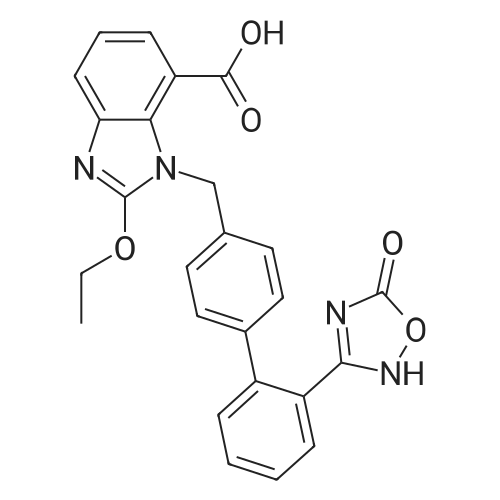
| 描述 | The numerous effects of Angiotensin II (AII), including its roles in vasoconstriction, secretion of aldosterone and vasopressin, cellular proliferation, and hypertrophy are dominantly mediated through the activation of the angiotensin type 1 (AT1) receptor, a member of the superfamily of G protein-coupled receptors. Azilsartan (AZL) is an AT1 receptor antagonist with IC50 of 2.6 nM. Pretreatment of AZL for 90 min inhibited the specific binding of 125I-Sar1-Ile8-AII to human AT1 receptors expressed in CHO cells in a concentration-related manner with an IC50 of 2.6 nM, indicating a high affinity for AT1 receptors. A potent inhibitory effect of AZL at AT1 receptors was maintained even 5 h after washout with an IC50 value of 7.4 nM. Pretreatment of intact COS-7 cells expressing human AT1 receptor with AZL for 2 h inhibited the accumulation of IP1 with IC50 value of 9.2 nM. Pretreatment with 0.1, 0.3, or 1 nM AZL for 30 min inhibited AII-induced aortic contraction in a concentration-related manner [7]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01715584 | Hypertension | Phase 4 | Recruiting | December 31, 2019 | Canada, Ontario ... 展开 >> London Health Sciences Centre - Victoria Campus Recruiting London, Ontario, Canada, N6A 5W9 Contact: Craig J Railton, MD, PhD 519 685 8500 ext 58525 Craig.Railton@lhsc.on.ca Principal Investigator: Craig J Railton, MD, PhD Sub-Investigator: Jonathan Fairbairn, BSc Sub-Investigator: George Nicoloau, MD Sub-Investigator: Robert Gros, PhD Sub-Investigator: Jason Franklin, MD Sub-Investigator: John Yoo, MD Sub-Investigator: Kevin Fung, MD Sub-Investigator: Anthony Nichols, MD Sub-Investigator: Danielle McNeil, MD 收起 << |
| NCT02235909 | Hypertension | Phase 3 | Recruiting | August 2020 | - |
| NCT00362115 | Hypertension | Phase 2 | Completed | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.19mL 0.44mL 0.22mL |
10.95mL 2.19mL 1.10mL |
21.91mL 4.38mL 2.19mL |
| 参考文献 |
|---|