| 生物活性 | |||
|---|---|---|---|
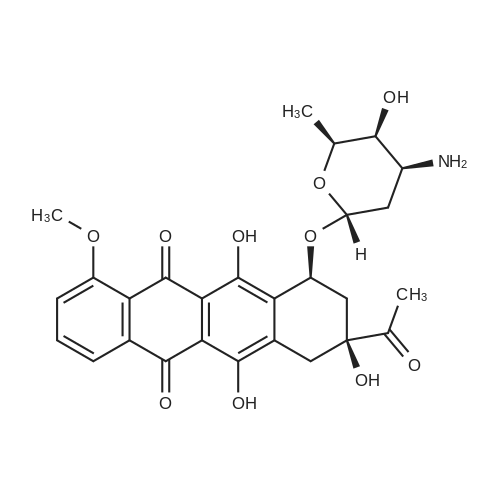
| 描述 | Daunorubicin, a topoisomerase II inhibitor, inhibits both DNA and RNA synthesis and inhibits DNA synthesis with Ki of 0.02 μM. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02198482 | Acute Myeloid Leukemia (AML) ... 展开 >> High-risk Myelodysplastic Syndrome (MDS) 收起 << | Phase 2 | Terminated(development program... 展开 >> of study drug volasertib was stopped by Boehringer Ingelheim due to manufacturing problems) 收起 << | - | Germany ... 展开 >> Hospital Aschaffenburg Aschaffenburg, Germany, 63739 Helios Hospital Bad Saarow Bad Saarow, Germany, 15526 Vivantes Hospital Am Urban Berlin, Germany, 10967 Vivantes Hospital Neukölln Berlin, Germany, 12351 Charite Berlin Campus Virchow Hospital Berlin, Germany, 13353 Knappschaftskrankenhaus Bochum-Langendeer Bochum, Germany, 44892 University Hospital Bonn Bonn, Germany, 53105 Community Hospital Braunschweig Braunschweig, Germany, 38114 Hospital Darmstadt Darmstadt, Germany, 64283 University Hospital Düsseldorf Düsseldorf, Germany, 40225 Hospital Essen, Protestant Hospital Essen-Werden Essen, Germany, 45239 Hospital Esslingen Esslingen, Germany, 73730 Malteser Hospital St. Franziskus Flensburg, Germany, 24939 Hospital Frankfurt-Höchst Frankfurt, Germany, 65929 Medical Care Unit Osthessen Fulda, Germany, 36043 University Hospital Gießen Gießen, Germany, 35392 Wilhelm-Anton-Hospital Goch Goch, Germany, 47574 University Hospital Göttingen Göttingen, Germany, 37075 University Hospital Hamburg-Eppendorf Hamburg, Germany, 20246 Asklepios Hospital Altona Hamburg, Germany, 22763 Hospital Hanau Hanau, Germany, 63450 KRH Hospital Siloah-Oststadt-Heidehaus Hannover, Germany, 30459 Hannover Medical School Hannover, Germany, 30625 SLK-Hospital Heilbronn Heilbronn, Germany, 74078 Marienhospital Herne Herne, Germany, 44625 University Hospital des Saarlandes Homburg/Saar, Germany, 66421 Community Hospital Karlsruhe Karlsruhe, Germany, 76133 University Hospital Schleswig-Holstein Kiel, Germany, 24105 Caritas Hospital Lebach Lebach, Germany, 66822 Hospital Lippe-Lemgo Lemgo, Germany, 32657 University Hospital Magdeburg Magdeburg, Germany, 39120 University Hospital Johannes Gutenberg Mainz Mainz, Germany, 55131 Johannes Wesling Hospital Minden Minden, Germany, 32429 Stauferklinikum Schwäbisch-Gmünd Mutlangen, Germany, 73557 Hospital Schwabing München, Germany, 80804 Hospital rechts der Isar München München, Germany, 81675 Hospital Oldenburg Oldenburg, Germany, 26133 Hospital Passau Passau, Germany, 94032 Hospital Stuttgart Stuttgart, Germany, 70174 Diakonie Hospital Stuttgart Stuttgart, Germany, 70176 Hospital Traunstein Traunstein, Germany, 83278 Mutterhaus der Borromäerinnen Trier, Germany, 54290 Hospital Barmherzige Brüder Trier Trier, Germany, 54292 University Hospital Tübingen Tübingen, Germany, 72076 University Hospital Ulm Ulm, Germany, 89081 收起 << |
| NCT02236013 | Acute Myeloid Leukemia | Phase 1 | Recruiting | July 2022 | United States, California ... 展开 >> Site US10003 Recruiting Los Angeles, California, United States, 90095 United States, Connecticut Site US10013 Recruiting New Haven, Connecticut, United States, 06520 United States, Illinois Site US10004 Recruiting Chicago, Illinois, United States, 60611 United States, Kansas Site US10009 Recruiting Westwood, Kansas, United States, 66205 United States, Maryland Site US10001 Recruiting Baltimore, Maryland, United States, 21231 United States, New York Site US10002 Recruiting New York, New York, United States, 10032 United States, Ohio Site US10014 Recruiting Cleveland, Ohio, United States, 44106 Site US10008 Withdrawn Columbus, Ohio, United States, 43201 United States, Oklahoma Site US10019 Recruiting Oklahoma City, Oklahoma, United States, 73104 United States, Pennsylvania Site US10006 Recruiting Philadelphia, Pennsylvania, United States, 19104 United States, Texas Site US10010 Recruiting San Antonio, Texas, United States, 78229 收起 << |
| NCT00037583 | Acute Myeloid Leukemia | Phase 2 | Completed | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.90mL 0.38mL 0.19mL |
9.48mL 1.90mL 0.95mL |
18.96mL 3.79mL 1.90mL |
| 参考文献 |
|---|