| 生物活性 | |||
|---|---|---|---|
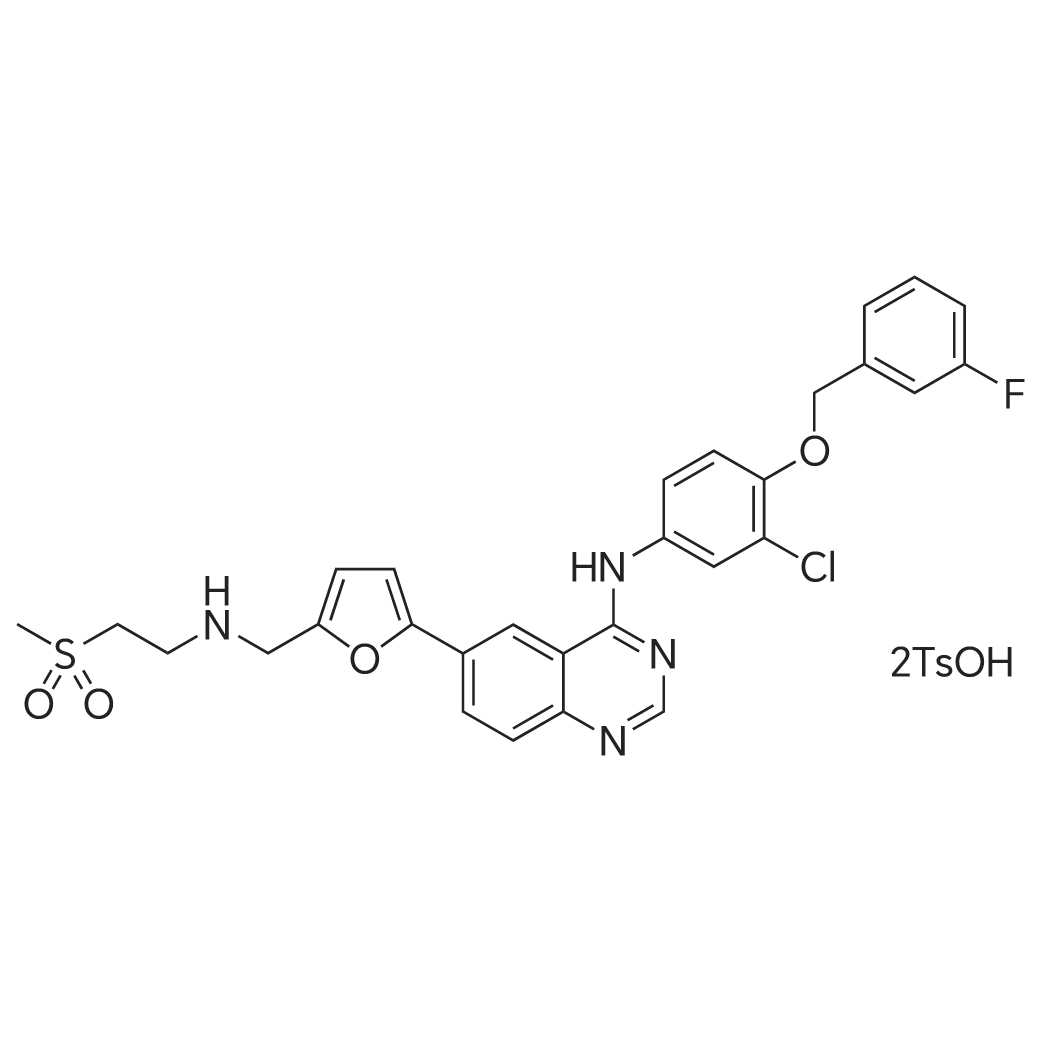
| 描述 | Deregulated expression of receptors EGFR and HER2, two closely related members of the ErbB family of transmembrane receptor tyrosine kinases, has been implicated in the development and malignancy of numerous types of human cancers, making them become a potential target for therapy. Lapatinib Ditosylate is the ditosylate form of lapatinib. Lapatinib is a potent and selective inhibitor of ErbB-2 and EGFR with IC50 values of 9.8nM and 10.2nM (measured by kinase activity assay), respectively. Treatment with lapatinib at concentration ranging in 0.03-10μM for 6h caused dose-dependent decrease of autophosphorylation of both EGFR and ErbB-2 in HN5 and BT474 cells, alone with the decreased phosphorylation level of the key signal transduction mediator AKT. Cell lines with overexpression of ErbB-2 or EGFR, including A431, HN5, BT474, N87, CaLu-3 and HB4a c5.2, were more sensitive to lapatinib with IC50 values ranging in 0.09-0.21μM 72h growth inhibition assays, showing the selective inhibition of cell growth by lapatinib. Induction of G1 arrest can be observed in HN5, EGFR-overexpressing cell line, treated with 1 or 10μM lapatinib. Apoptosis can be significantly induced by 10μM lapatinib after 72h in BT474 cells. Oral administration of lapatinib at dose of 100mg/kg twice daily for 21 days achieved a complete inhibition of tumor growth in both BT474 and HN5 human tumor xenografts[1]. | ||
| 作用机制 | Lapatinib is an ATP-competitive inhibitor of both EGFR and HER2.[1] | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| 22Rv1 cell | Cytotoxicity assay | 4 days | Cytotoxicity against human 22Rv1 cells after 4 days by propidium iodide staining-based fluorometric analysis, IC50=6.06 μM | 22169601 | |
| A253 cell | Growth inhibition assay | Inhibition of human A253 cell growth in a cell viability assay, IC50=2.0483 μM | SANGER | ||
| A388 cell | Growth inhibition assay | Inhibition of human A388 cell growth in a cell viability assay, IC50=2.0483 μM | SANGER | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00849329 | Neoplasms, Breast | Phase 1 | Completed | - | United States, South Carolina ... 展开 >> GSK Investigational Site Greenville, South Carolina, United States, 29605 Korea, Republic of GSK Investigational Site Seoul, Korea, Republic of, 135-710 GSK Investigational Site Songpa-gu, Seoul, Korea, Republic of, 138-736 Spain GSK Investigational Site Hospitalet de Llobregat (Barcelona), Spain, 08907 收起 << |
| NCT00486954 | Neoplasms, Gastrointestinal Tr... 展开 >>act 收起 << | Phase 3 | Completed | - | China, Guangdong ... 展开 >> GSK Investigational Site Guangzhou, Guangdong, China, 510060 China GSK Investigational Site Beijing, China, 100021 GSK Investigational Site Beijing, China, 100071 GSK Investigational Site Shanghai, China, 200032 Japan GSK Investigational Site Tokyo, Japan, 113-8677 Korea, Republic of GSK Investigational Site Hwasun, Korea, Republic of, 519-809 GSK Investigational Site Seongnam-si Gyeonggi-do, Korea, Republic of, 463-707 GSK Investigational Site Seoul, Korea, Republic of, 110-744 GSK Investigational Site Seoul, Korea, Republic of, 120-752 GSK Investigational Site Seoul, Korea, Republic of, 135-710 Taiwan GSK Investigational Site Kaohsiung, Taiwan, 807 GSK Investigational Site Niaosong Township, Kaohsiung, Taiwan, 833 GSK Investigational Site Tainan County, Taiwan, 736 GSK Investigational Site Tainan, Taiwan, 704 GSK Investigational Site Taipei, Taiwan, 100 GSK Investigational Site Taipei, Taiwan, 112 GSK Investigational Site Tau-Yuan County, Taiwan, 333 收起 << |
| NCT00486954 | - | Completed | - | - | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.08mL 0.22mL 0.11mL |
5.40mL 1.08mL 0.54mL |
10.81mL 2.16mL 1.08mL |
| 参考文献 |
|---|